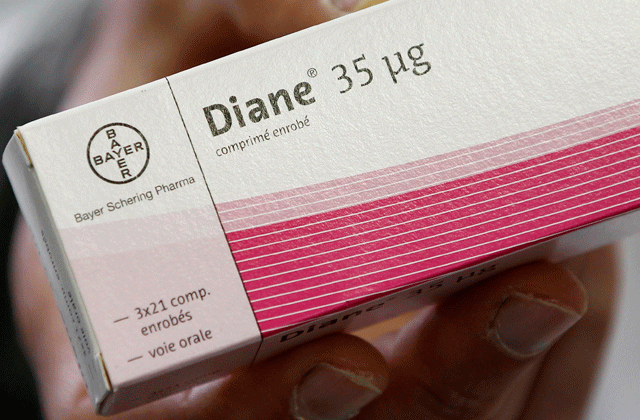
Dubai: The Ministry of Health has called on the local health authorities and directors of medical districts, public and private hospitals and private clinics to temporarily suspend the usage of Diane 35 due to its side effects.
The suspension is based on the recommendations of the French Drugs Agency "ANSM". Studies showed that their usage may cause blood clots, the ministry said on Tuesday.
Dr. Amin Hussain Al Amiri, Assistant Undersecretary for Medical Practices and Licensing and Chairman of the higher committee for registration and pricing, said: "The ministry recommended the temporary suspension of the above mentioned drug and all similar products in addition to the importation and marketing of the drug until the final evaluation of the product is done by international health authorities.”
He also stated that patients using the drug should not stop using the medicines independently, but consult their doctor. He said doctors must not prescribe new prescriptions or renew the prescription of the medicine.
"The product is registered in the Ministry of Health according to the rules and regulations of the drugs registration operations and process. However, this does not prevent the ministry from using the correct safe and healthy procedures in case any side effects appear due to the usage of any medicine", Al Amiri said.
The official explained that ANSM discovered blood clots complications which are already mentioned in the drug's internal newsletter. With the increasing number of these cases, the French agency decided to suspend this product.
The European Drugs Authority is currently examining and studying the product in its laboratories and coordinating with the UAE Ministry of Health regarding to the continuation of the product or withdrawing it completely from the markets.
Al Amiri explained that incase of any side effects or accidents, the community members are requested to fill in the form available on the website, www.cpd-pharma.ae.
In case of emergencies or side effects of the product, one can reach to registration and pharmaceutical control administration on the following direct telephone numbers: 026117391 or 026117318, fax number: 02-6313742 or email address: pv@moh.gov.ae.