Ministry of Health withdraws medicine batch in UAE
A batch of medicine was withdrawn from UAE market for not meeting specifications
Also In This Package
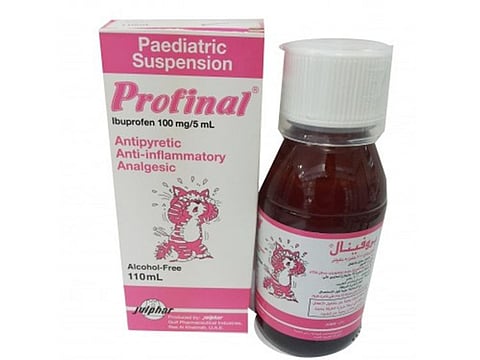
Dubai: The Ministry of Health and Prevention (MoHAP) issued a circular to withdraw batch No.0621 of the pharmaceutical product Profinal 100 mg/ 5ml.
Profinal is a paediatric oral suspension mainly prescribed for children whenever a rapid effect is required to lower fever and relieve pain.
The product was manufactured by the Ras Al Khaimah-based company Julphar Gulf Pharmaceuticals.
Also Read: UAE withdraws popular diabetes medicine
Based on the ministry’s laboratory results, the medicine was deemed unfit as it did not meet the standard specifications.
In a circular issued by Dr Ameen Hussain Al Amiri, assistant undersecretary for Licencing and Public Health Policy at the ministry, the batch was withdrawn from the local market.
The product, which was registered with the Medication Department at MoHAP, will be withdrawn from public and private health sectors immeditaley. The Ministry ordered all healthcare practitioners not to prescribe the medicine.
Sign up for the Daily Briefing
Get the latest news and updates straight to your inbox



