Highlights
- Hair growth drug baricitinib has received regulatory approval to treat alopecia.
- Alopecia is an “auto-immune” disease, the second-most common form of hair loss affecting an estimated 147 million people worldwide.
- The approval came following decades of research, including recent randomised trials involving 1,200 patients.
There’s hope for people who suffer from hair loss. Alopecia areata (or just alopecia) affects tens of millions of people.
The condition usually causes hair loss on the scalp. Some patients also experience facial and body hair loss, with dreadful consequences — especially for children stricken by alopecia.
Scientists have known the condition for years as an “autoimmune” disease.
No drug was available to provide relief.
Now, there’s one.
The drug has been approved June 13 for treating alopecia following extensive studies going back decades. Two recent clinical trials conducted on more than 1,200 volunteers showed clear benefits — with minimal side-effects, according to the US Food and Drug Administration. Study results were significant: they're the first such Phase 3 trials of any treatment for alopecia.
Here’s what you should know:
What is the drug called?
It is called baricitinib, under the brand name Olumiant. It's an oral tablet.

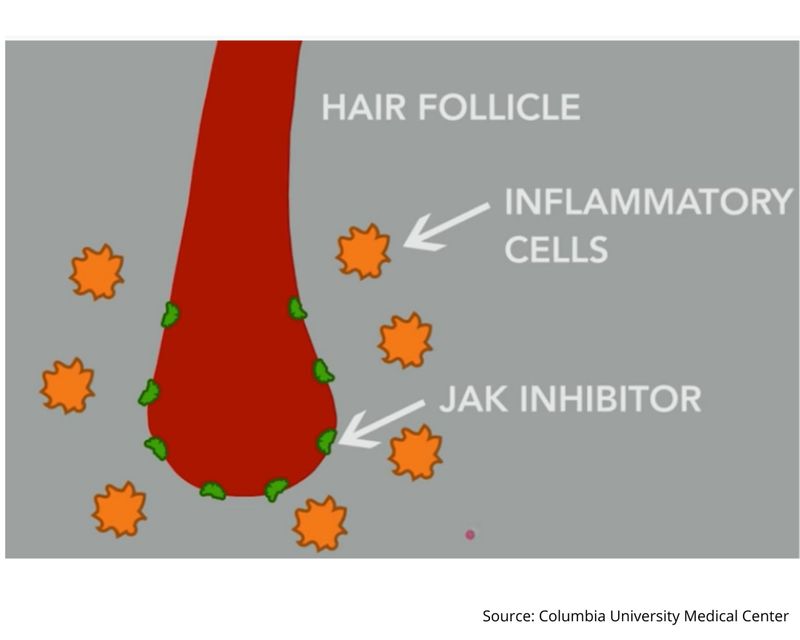
Olumiant is known as a Janus kinase (JAK) “inhibitor”, known to block the activity of one or more of a specific family of enzymes, interfering with the pathway that leads to inflammation.
147 m
Estimated number of people worldwide who have or will develop alopecia areata at some point in their lives.What does it do?
In a nutshell, the treatment regrows hair by blocking the immune system from attacking hair follicle.
When was it approved for alopecia, and where?
It was approved June 13, 2022, by the US Food and Drug Administration.
Is it available now?
It is a prescription drug and is already available on the market — for the treatment of rheumatoid arthritis and other autoimmune diseases.
Pfizer and Concert Pharmaceuticals are also applying for regulatory approval of their own “JAK inhibitors”.
When does alopecia begin?
It can start at any age. Children and teenagers can have it. However, most people develop it by 30 years of age.
When did the first sign emerge that the formula may work?
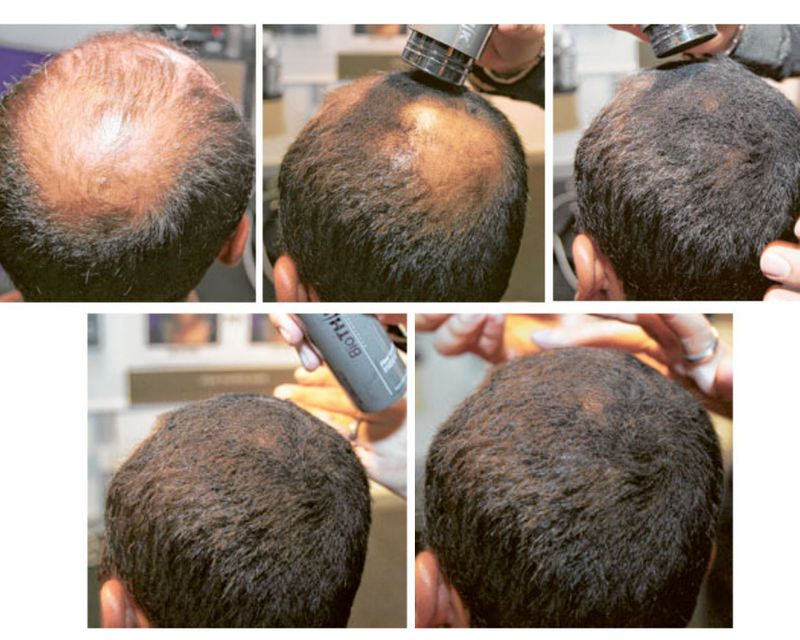
In 2016 a small trial involving 12 people, researchers researchers from Columbia University Irving Medical Center (CUIMC) found that 75 per cent of patients with moderate to severe alopecia had significant hair regrowth after treatment with ruxolitinib.
By the end of treatment, average hair regrowth among the patients was 92 per cent. Though the study’s findings were based on a small sample, it was significant as the Columbia researchers had identified the specific immune cells and the dominant inflammatory signalling pathways responsible for attacking the hair follicle in alopecia.
This “auto-immune” attack was found to be responsible for putting hair follicle into a “dormant” state.
What happened next?
Fast forward six years.
The efficacy and safety study of Olumiant in alopecia was led by Yale University researchers in two randomised, double-blind, placebo-controlled trials. It was conducted among a much bigger number of patients (1,200) who had at least 50 per cent scalp hair loss for more than six months.
The patients received either a placebo, 2 milligrams of Olumiant, or 4 milligrams of Olumiant every day.
How was the efficacy measured?
To measure the drug’s efficacy, both trials counted the proportion of patients who achieved at least 80% scalp hair coverage at week 36.
How effective is baricitinib (Olumiant)?
In the first trial, 22% of the 184 patients who received 2 milligrams of baricitinib (Olumiant) and 35% of the 281 patients who received 4 milligrams of Olumiant achieved adequate scalp hair coverage.
This is compared to 5% of the 189 patients who received a placebo, according to the US Food and Drug Administration.
Also in the second trial, 17% of the 156 patients who received 2 milligrams of Olumiant and 32% of the 234 patients who received 4 milligrams of Olumiant achieved adequate scalp hair coverage, compared to 3% of the 156 patients who received a placebo, FDA reported.

Do other diseases trigger hair loss (alopecia areata)?
According to the American Academy of Dermatology, other common triggers for alopecia areata include:
- Atopic dermatitis
- Asthma
- Hay fever
- Thyroid disease
- Vitiligo
- Down syndrome
Research shows that people who have one of these diseases are more likely to get alopecia areata.
What were the side effects?
Patients in the Lilly study experienced relatively mild side effects — including:
- Small increased risk of acne
- Urinary tract infections
- Other infections
These side effects, however, were considered “mild”, and were easily treatable or improved without treatment.
Why is the approval significant?
Alopecia affects millions of people. It's the first time a drug for alopecia has received regulatory approval. For certain patients, FDA approval is important for insurance coverage of these expensive drugs.
How much is the drug Olumiant (baricitinib)?
In the US, Olumiant has a list price of nearly $2,500 a month, according to US media reports.