India: World's biggest vaccine maker sees malaria shot saving kids' lives
Malaria has been one of the world's neglected diseases with 96% of deaths in Africa
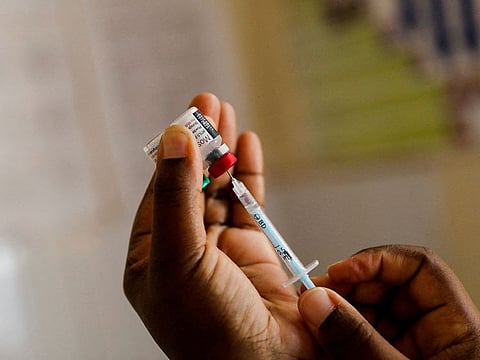
Serum Institute of India Ltd., the world's biggest vaccine maker, said its shot that gained a World Health Organization recommendation has the potential to significantly reduce fatalities from malaria, one of the globe's most deadly diseases, particularly among young children in Africa.
The vaccine developed with researchers at the University of Oxford, known as R21, was recommended Monday by the WHO for use in Africa for children under five years old, the age group and continent that bear the brunt of a disease that kills more than 600,000 people a year.
"We can protect the children and lives in Africa against this disease as soon as possible," said Adar Poonawalla, Serum's chief executive officer, in an interview. "It has the potential, if rolled out successfully, to save perhaps the most lives."
Malaria has been one of the world's neglected diseases with 95% of cases and 96% of deaths occurring in Africa, many of them in the poorer countries of the continent. The lack of a lucrative market has meant relatively little impetus for development of a shot.
It's also been a tricky target for vaccine makers. The parasites that cause the deadly disease are prone to mutations that allow them to develop resistance to treatments. BioNTech SE, which developed the highly potent mRNA Covid vaccine in partnership with Pfizer Inc, is also working on a vaccine candidate for malaria. Last year, the WHO formally endorsed Mosquirix, the world's first vaccine for the disease that was developed by GSK Plc and its partners.z
Approval of Serum's shot should ease supply constraints as only 18 million doses of GSK's are available through 2025.
Serum's immunization uses an adjuvant developed by Novavax Inc. to help raise an immune response to malaria and specifically targets a version of the parasite that's prevalent in Africa. The firm can produce 100 million doses of the vaccine a year, with 20 million already in stock, and expects to start distribution next year, Poonawalla said. Production capacity will be doubled over the next two years.
Twenty-eight African countries have expressed interest and 18 are ready to receive the vaccine, the CEO said. Shipments will primarily be distributed via Unicef and Gavi, the Vaccine Alliance, he said, as many countries won't have the money to pay for them.
The recommendation is a required step for the WHO to prequalify the vaccine through assessment of data relating to its efficacy and safety. Prequalification is a requirement for United Nations agencies such as Unicef and Gavi to deploy it.
The cost will be less than $4 a shot, cheaper than what is currently available, with three doses to be taken by children between five and 36 months of age with a booster to be taken a year later. The vaccine's efficacy was shown in a clinical trial to be as high as 80% a year after the fourth dose was administered.
Sign up for the Daily Briefing
Get the latest news and updates straight to your inbox






