COVID-19: Plasma therapy vs stem cell therapy, what’s the difference?
2 promising therapies among weapons dispatched against coronavirus
Also In This Package
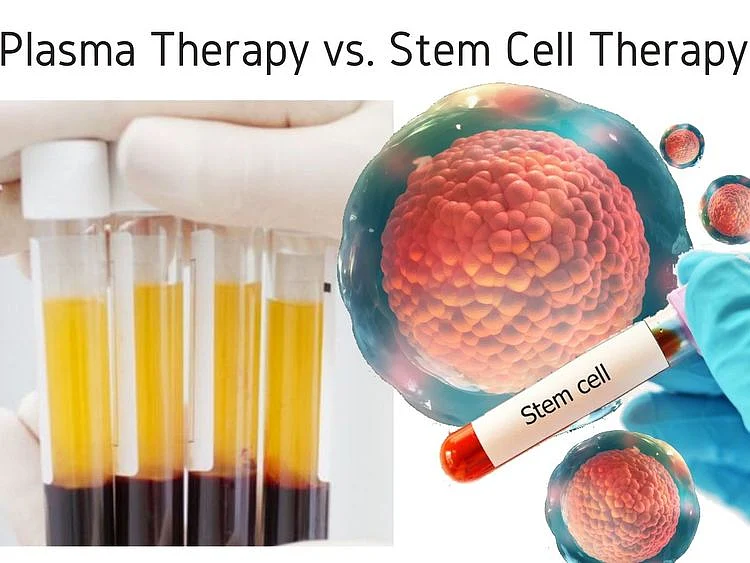
DUBAI: Plasma and stem cell (SC) therapies are two of the emerging star treatments being used in the fight against the SARS-CoV-2 virus.
Against COVID-19, they're considered stop-gap measures, while the world awaits a vaccine. Both, however, have proven effective against severe cases infections caused by the novel coronavirus, which has already killed over 502,000 and infected 10.1 million as of Monday (June 29, 2020).
Plasma and SC therapies have similarities, as well as obvious differences. We outline them below:
Q: What are their similarities?
Both plasma and stem cell therapies rely on rejuvenating damaged body tissue. They both form part of what's described as “regenerative medicine”, a fast-emerging branch of medical science involving techniques that help restore the function of tissues or organs.
Being “regenerative” treatments (or therapies), they encourage your body to use its natural abilities to heal injuries or other types of tissue damage or inflammation.
The journal Platelets refer to platelet-rich plasma (PRP) and stem cell (SC) therapies as the "mainstream medical technologies" to repair and rejuvenate a damaged tissue or organ caused by injury or chronic diseases.
Q: What are the key differences?
Plasma-rich platelets are components of blood that contain platelet concentrations above the normal level.
Platelets are the frontline workers in carrying out a healing response to injuries. They release growth factors for tissue repair. Plasma therapy uses the liquid portion of blood (plasma, yellowish) which includes a higher concentration of platelets — the part of blood that contributes to clotting and healing.
Stem cells, on the other hand, are “generic” cells. They are the prime cells -- unspecialised, undifferentiated, immature cells. Based on specific stimuli, they can divide and differentiate into specific type of cells and tissues.
Q: What are stem cells?
Stem cells are the basic, “generic” building blocks of life. In a sense, they unspecialised, undifferentiated, immature cells. They can divide and differentiate into specific type of cells and tissues, based on based on specific stimuli.
It’s this ability to differentiate into other types of cell that make stem cells interesting to medical science.
In adults, they are usually obtained from bone marrow. In infants, stem cells are usually taken from the umbilical cord.
Scientists have found stem cells present in blood vessels, the brain, skeletal muscles, skin and the liver.
They can be difficult to find and work with. Stem cells are also categorised by their potential to differentiate into different cell types. These include, “pluripotent” and “multipotent” stem cells.
Q: What are the advantages of stem cell-based therapies?
SCs are generic (or primitive) cells obtained either from embryos or from the adult tissues, that have the capacity of self-renewal and can differentiate into as many as 200 different cell types of the adult body.
SC also produces certain growth factors and “cytokines” that accelerate the healing process at the site of tissue damage. Cytokines are secreted by certain cells of the immune system and have an effect on other cells. SC is used to treat degenerative and inflammatory conditions by replacing the damaged cells in virtually any tissue or organ, where PRP applications serve no benefit.
Q: How is stem cell therapy helping fight COVID?
In the UAE, a medical research team has developed a first-in-the-world technique using “inhaled” stem cells that harvested from the patients themselves. Following the initial success of the technique in 73 patients, the procedure has been ramped up.
As of June 26, 2020, the SC nebuliser type therapy, called UAECell19, has treated more than 2,000 severe cases of COVID-19 patients, with 1,200 now out of the hospital.
In May, the ADSCC team, led by Dr Yendry Ventura, unveiled the new treatment. The UAECell19 is an autologous (cells obtained from the same individual) stem cells therapy which helped cut hospital stay from 22 days to six, relative to patients who were given standard treatment.
Q: What's the record of UAECell19 stem cell therapy?
According to the Abu Dhabi team, patients given the stem cell therapy were up to 3.1x more likely to recover in less than seven days compared to those given standard treatment.
Researchers also stated that 67% of the patients who received the stem cells treatment owed this recovery to the new treatment.
ADSCC has secured a patent for UAECell19. Protection of the intellectual property rights for the therapy opens doors for it to be shared more widely so more patients can benefit.
Q: What is convalescent plasma?
Plasma is the liquid portion of whole blood. It is composed largely of water and proteins, and it provides a medium for red blood cells, white blood cells and platelets to circulate through the body.
“Convalescent” refers to a person recovering from an illness or medical treatment.
Convalescent plasma, also known as immunoglobulins, is plasma taken from the blood of a person who has recovered from a disease.
Research shows that recovered COVID-19 patients develop antibodies in the blood against the virus. Antibodies are proteins that might help fight the infection.
Q: What are platelets?
Platelets, a component of the blood, are repair agents. They are frontliners in the healing response to injuries. Platelets are also called “thrombocytes”, blood cells that trigger blood clotting and other necessary growth healing functions.
PLATELET FACTS
[] A normal platelet count is 150,000 to 450,000 platelets per microliter of blood.Your risk for bleeding develops if a platelet count falls below 10,000 to 20,000. [] When the platelet count is less than 50,000, bleeding is likely to be more serious if you're cut or bruised. [] Some people make too many platelets. They can have platelet counts from 500,000 to more than 1 million.
Q: What is platelet-rich plasma (PRP)?
PRP is a component of blood that contains platelet concentrations above the normal level -- usually five times higher concentrations of platelets above the normal values. It includes platelet-related growth factors and plasma-derived fibrinogen (a blood plasma protein that's made in the liver), among others.
Q: Has it been used to treat other diseases?
Yes. Convalescent plasma has been used as a last resort to improve the survival rates of patients with SARS (2003), as well as the "Spanish Flu" (1918-1920), as well as other infectious diseases. The Lancet cites several studies that showed a shorter hospital stay and lower mortality in patients treated with convalescent plasma than those who were not treated with it.
It's been gaining ground in the COVID-19 fight around the world. In the last few weeks, convalescent plasma therapy has helped treat at least 170 patients at the Infectious Disease Department at Rashid Hospital in Dubai.
THROMBOCYTOPENIA
[] A condition when your bone marrow makes too few platelets, or your platelets are destroyed. If your platelet count gets too low, bleeding can occur under the skin as a bruise. [] This can be caused by many conditions — by medicines, cancer, liver disease, pregnancy, infections (including COVID-19), and an abnormal immune system.
Q: Why is convalescent plasma important?
If you are one of the thousands of patients fully-recovered from COVID-19, you may be able to help patients currently fighting the infection — by donating your plasma. That's because you fought the infection, your plasma now contains COVID-19 antibodies.
These antibodies provided one way for your immune system to fight the virus when you were sick, so your plasma may be able to be used to help others fight off the disease.
Q: I have fully recovered from COVID-19. Am I eligible donate plasma?
Yes, you are. Health authorities, including the US Food and Drug Administration, encourage people who have fully recovered from COVID-19 for at least two weeks to consider donating plasma.
You can help save the lives of other patients.
However, you must first undergo some tests to check if you're eligible to meet donor criteria. Doctors will determine that. COVID-19 convalescent plasma must only be collected from recovered individuals if you are eligible.
A lab test must document a prior diagnosis of COVID-19. In general, FDA protocol requires individuals to have complete resolution of symptoms for at least 14 days prior to donation.
Q: Is a negative lab test for COVID-19 a must before being considered to donate blood plasma?
No. The FDA guideline states: “A negative lab test for active COVID-19 disease is not necessary to qualify for donation.”
Q: I haven’t had COVID-19. What can I do to help?
You should consider donating blood. One blood donation can save up to three lives.
Also Read
How you can donate blood in the UAENetwork Links
GN StoreDownload our app
© Al Nisr Publishing LLC 2026. All rights reserved.