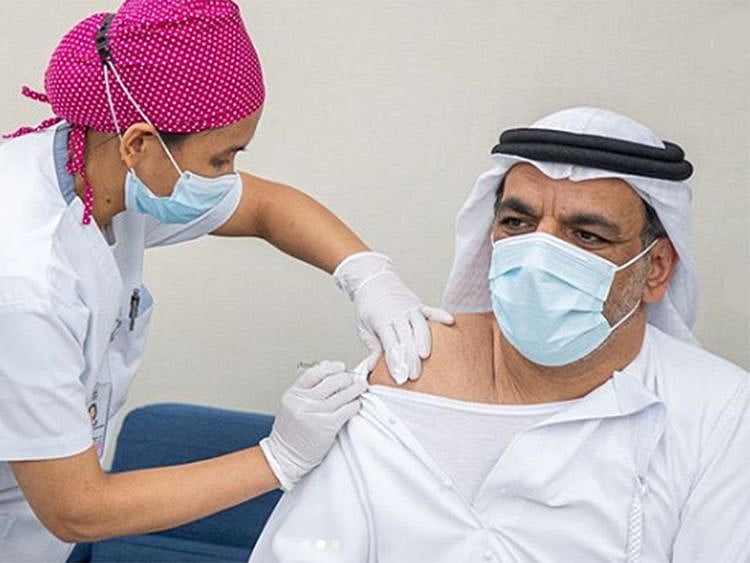
COVID-19: Analysis of UAE vaccine trial results begins
First 100 volunteers have completed more than 42 days since first shot of vaccine
Also In This Package
UAE parents, students go shopping for the new term
10 tips to keep your dog happy in the summer
UAE: Where is my refund for a cancelled flight?
IPL in UAE: KXIP and Rajasthan get down to training
Dubai schools disinfect buses ahead of new term
Reader's photos: Desert safari tour
Emirati Women’s Day: Celebrating women in every field
Abu Dhabi: The results of the first 100 volunteers in the UAE vaccine trial are now beginning to be analysed, a top official has said.
Speaking to Gulf News, Dr Jamal Al Kaabi, undersecretary at the Abu Dhabi Department of Health (DoH), said the analysis of blood test results has started, including the results he himself provided as the second volunteer of the vaccine trials.
Also Read
#InternationalDogDay: 10 tips to keep your dog happy in the summerGMC brings Sierra CarbonPro bed to the Middle EastPhotos: Gulf News reader shares pictures of the beautiful birds freely roaming in The Gardens Jebel Ali Dubai Emirati Women’s Day 2020: Celebrating women in every field“The tests of this double-blinded study are now being studied as more and more data is accumulated,” Dr Al Kaabi said.
Wednesday, August 26, marked 42 days since the start of vaccine trials in the UAE.
Inactivated vaccine trials
Dr Al Kaabi was one of two senior officials who kicked off the vaccine trials, dubbed 4Humanity, on July 16, with the DoH chairman, Abdullah Al Hamed, recorded as the first volunteer.
The vaccine is the first inactivated vaccine in the world to enter Phase III trials. It has been developed by Chinese pharmaceutical giant, Sinopharm China National Biotec Group. During its first two trial phases in China, all participants successfully generated antibodies when two shots of the vaccine were delivered 28 days apart.
In the UAE, the trials are being sponsored by Abu Dhabi-based artificial intelligence and cloud computing firm, G42, and they are being overseen by the DoH and the UAE Ministry of Health and Prevention. Abu Dhabi’s public health provider, the Abu Dhabi Health Services Company (Seha), is carrying out the medical aspects of the trial.
Double-blinded study
Dr Al Kaabi said the trial volunteers received one of three types of vaccine shots – Strain 1, Strain 2 or a placebo.
As a double-blinded study, neither the researcher nor the doctor will know what treatment was administered to a particular patient. This type of research is carried out to eliminate any potential baises the researcher or volunteer may have.
“Statistical analysis will now be carried out to see any correlations between the shots and any impact they have had. It depends on the [vaccine] developer, but the aim is typically to achieve a four-fold increase in immunity from the baseline,” Dr Al Kaabi said.
More than 15,000 volunteers have already signed up for the trials in the UAE.
Sign up for the Daily Briefing
Get the latest news and updates straight to your inbox
Network Links
GN StoreDownload our app
© Al Nisr Publishing LLC 2026. All rights reserved.