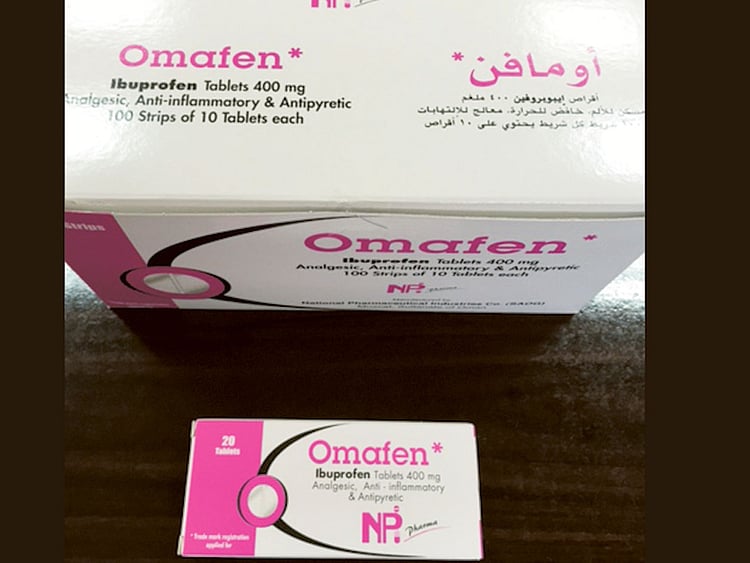
Dubai: The Ministry of Health and Prevention has withdrawn three batches of 400mg tablets and oral suspension of Omafen over safety concerns.
This follows an order issued by the General Directorate of Pharmacy in Oman where the medicine is routed as its physical varieties were mismatched and pills were found to contain traces of aluminium.
The medicine is a brand for the generic drug Ibuprofen, which has anti-inflammatory and pain-killing qualities and is used to reduce high temperature.
Dr Ameen Al Amir, assistant undersecretary for medicine practice and licence sector at the ministry, said: “The ministry has ordered to withdraw these three batches as patient safety is our priority and medicines have to comply to international pharmaceutical manufacturing standards.” He also added that alternative medicines were available in the UAE and the other batches of the medicine were safe for use.
The local agent distributing Omafen 400mg was ordered to recall the batch and stop distribution of the drug from Oman.
The Ministry has asked the agent to ensure the safety of future shipments of fresh batches of the medicine.
Meanwhile, several other formulations of Ibuprofen with the same potency manufactured locally by global pharmaceutical companies are available in the UAE.
Sign up for the Daily Briefing
Get the latest news and updates straight to your inbox
Network Links
GN StoreDownload our app
© Al Nisr Publishing LLC 2025. All rights reserved.