Geneva: The World Health Organization (WHO) said on Wednesday that it had prequalified Takeda Pharmaceuticals' dengue vaccine.
TAK-003 is the second dengue vaccine to be prequalified by WHO. Developed by Takeda, it is a live-attenuated vaccine containing weakened versions of the four serotypes of the virus that cause dengue.
WHO recommends the use of TAK-003 in children aged 6–16 years in settings with high dengue burden and transmission intensity. The vaccine should be administered in a 2-dose schedule with a 3-month interval between doses.
The WHO prequalification list also includes CYD-TDV vaccine against dengue developed by Sanofi Pasteur.
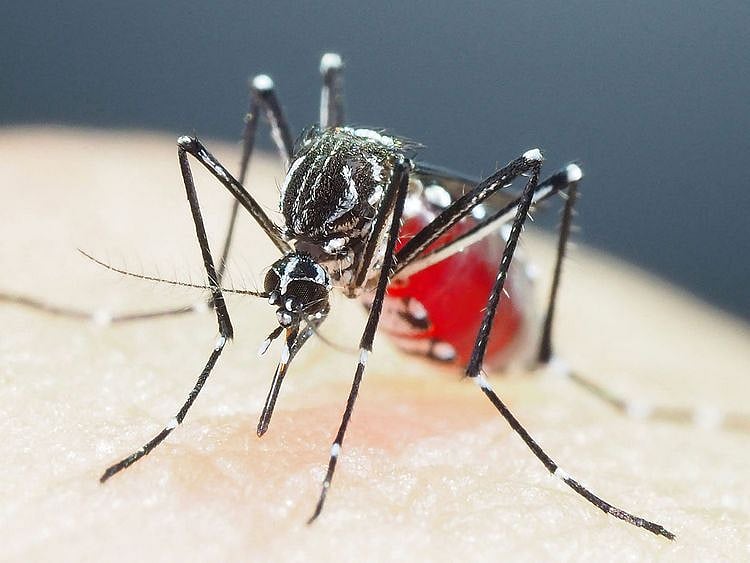
Dengue is a vector-borne disease transmitted by the bite of an infected mosquito. Severe dengue is a potentially lethal complication which can develop from dengue infections.
Sign up for the Daily Briefing
Get the latest news and updates straight to your inbox
Network Links
GN StoreDownload our app
© Al Nisr Publishing LLC 2026. All rights reserved.