COVID-19 vaccine trial: They deliberately infected themselves
How volunteers are chosen from across the world to take an unproven jab
Highlights
- At least 25 coronavirus candidate vaccines are already running human trials, with several in final Phase III
- There are more than 120 candidate vaccines listed by the WHO
- Here's how participants are picked for each trial phase, monitored and their progress electronically recorded
- There are protocols that must be adhered to, regulatory oversight to be followed
- Sometimes, vaccine inventors deliberately infect themselves to prove their concoction works
Also In This Package
Explained: COVID-19 test for entering Abu Dhabi
First look: Places of worship in the UAE are now open
COVID-19: Can my new employee fly in after July 7?
Pictures: How India's largest slum beat back a pandemic
Indians opt for alternative careers during COVID-19
COVID-19 Thailand: Schools reopen with isolated desks
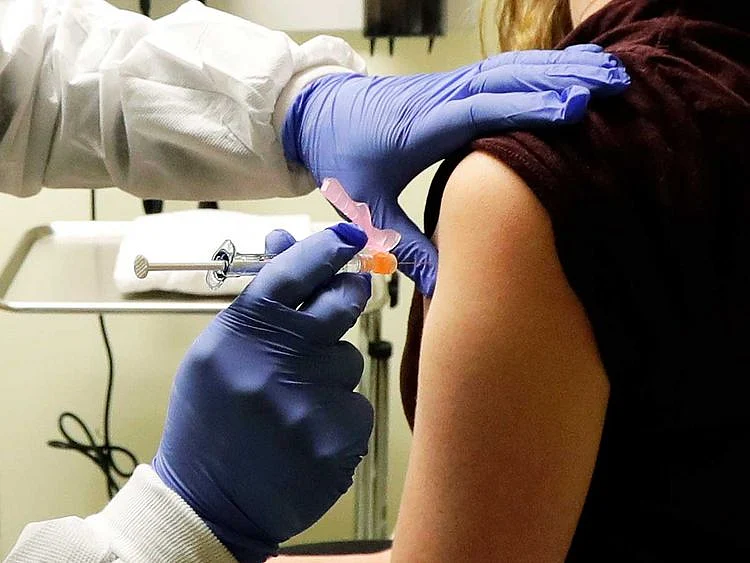
DUBAI: Participating in a vaccine trial using an "attenuated-live" vaccine means deliberately infecting yourself.
It's a form of altruism, backed by informed consent on the part of participants. It's a calculated risk-taking for the common good.
And that's what thousands of people are doing in six continents, including Phase III coronavirus vaccine trials in the UAE.
Vaccine trials used to be one-man shows, with inventors sometimes deliberately infecting themselves to prove their concoction works.
Think of Dr. Hilary Koprowski, who invented the polio vaccine: He drank the concoction in 1948 — the first trial of an attenuated "live" poliomyelitis virus — and won a Novel Prize three years later for his feat.
Race or harmony?
The times they are a-changin': Today, the huge number of coronavirus vaccine trials are more like a global harmony. At least that’s the ideal. There's a full array of technologies or platforms used, hundreds of scientific teams at work, and thousands of volunteers.
Tough rules apply to these trials, with oversight from health authorities. Every part of it is recorded and open to verification. Except for patient/volunteer confidentiality, the trials are run by open science. Vaccine developers must hit the right notes.
If your candidate vaccine does not make the cut, thanks very much. It's out. For after all, a vaccine means injecting a foreign substance into a huge number of healthy people. It better work — or the vaccine bombs.
Now, bigger trials are about to prove if any shot works in real life. The end of these trials will be the flavour of Summer 2020, and the subsequent seasons.
Also Read
Made-In-India COVID-19 vaccines: What happens nextAstraZeneca COVID-19 vaccine likely to protect for a year - CEO Anti-COVID-19 weapons: Teaching old drugs new tricksHere’s the good news
It's different this time, which brings good tidings, sort of, in the fight against COVID-19. Hundreds of scientific teams and thousands of volunteers are doing marathon research projects to bring out a good jab.
At least three are currently in Phase III trials, while many are either in Phases I or II, or somewhere in between.
What is the best possible COVID-19 vaccine?
One that is 100% effective and 100% safe.
Even if some vaccines may have advanced through the trials, others that have lagged behind may be simply more effective than the front-runners. Scientists say it’s not always clear why this is so, though they surmise it could be due to any of the following:
How many participants are involved in trials?
There are literally thousands of people who had been given different versions of the vaccine in six different continents. With different "platforms" used (live-attenuated, inactivated vaccines, subunit, RNA, DNA, conjugate vaccines, and toxoid vaccines), the outcome is one of the most-awaited moments of the new decade.
Each preparation will be given to a wide geographic area among thousands of recipients, based on different phases:
Phase I trials check the initial safety of a vaccine — at different doses — on a small number of healthy volunteers, from 20 to 80. This is done in a relatively small number of people.
Phase II expands on the first this to further validate the vaccine’s efficacy. This is where researchers check the presence of antibodies or other immunity markers in the blood of the participants that can neutralise the targeted pathogen.
Phase III uses more extensive metrics and a bigger number of volunteers — usually people from different age groups, and in the thousands. This phase tests the level of protection people get from a specific dosage. The immunity of those given the shot is compared with those who receive a placebo.
Phase IV trial is the real "acid test" of a vaccine, which can only come once approved and distributed widely. This trial would be post-rollout phase, when the shot is given to the wider community.
LIVE-ATTENUATED VACCINE VS 'KILLED' VIRUS VACCINE
Live vaccines use a weakened (or "attenuated") form of the pathogen that causes a disease. Because these vaccines are so similar to the natural infection that they help prevent, they create a strong and long-lasting immune response. Just 1 or 2 doses of most live vaccines can give you a lifetime of protection against a germ and the disease it causes. "Attenuation" alters and reduces the virulence of a pathogen, but still keeps it viable. It becomes harmless or less virulent. On the other hand, an "inactivated" vaccine uses the "killed" version of the disease-causing pathogen. Inactivated vaccines usually don’t provide immunity (protection) that’s as strong as live vaccines. So you may need several doses over time (booster shots) in order to get ongoing immunity against diseases. [Source: https://www.vaccines.gov/basics/types]
How clinical trial starts and how participants are selected
Pre-clinical trials usually involve animal studies. Those with the most promising possibilities are moved into human clinical trials.
In clinical trials, participants are enlisted to test an unproven medicine/vaccine, alongside a placebo – a preparation (inactive susbtance, usually starch or sugar) that has no therapeutic effect.
First, the volunteers are asked to sign a consent form. That way, they know fully well that they are part of an experiment and the many unknowns.
Clinicians involved in the trials follow them, and may stop the trial at any time, if the negative effects far outweigh the potential benefits. The Phase III part of the COVID-19 vaccine should be randomised, placebo-controlled and double-blind — the gold-standard of trials.
During a trial, more and more information is gained about the potential treatment, its risks and how well it may or may not work, along with aspects related to quality of life.
A research team follows a trial plan following the Good Clinical Practice (GCP) guidelines regulators require in order to protect patient safety. A trial plan outlines the following:
The Phase III part of the COVID-19 vaccine is usually randomised, placebo-controlled and double-blind — the gold-standard of trials on new therapies.
Are there ethical principles that govern clinical trials?
Yes. Ethical clinical research is guided by the principles of nonmalificence, respect, beneficence and justice.
Non-malificence
It's the duty to cause no harm (Hippocratic Oath).
Respect
This is embodied in informed consent, dictating that information is exhaustive and provided in a manner that is understandable, that the subject’s cooperation is voluntary, and that all information pertaining to the subject is held in confidence.
Beneficence
This is demonstrated by a thorough risk/benefit assessment, recognizing that benefits can be direct, collateral, and/or altruistic. Risks are considered in physiologic, psychological, and socioeconomic terms. For a clinical trial to be considered ethical, there must exist a sufficient body of scientific/medical evidence to justify exposure of individuals to the risks of the trial.
Justice
This takes into account all the processes by which populations are selected for study to ensure that the results benefit the community, avoid exploiting vulnerable populations, and include individuals who may be likely to benefit.
What about adherence to legal, ethical standards?
This is also called oversight. This helps ensure the integrity of the trial, in order to safeguard its scientific validity and protect participating individuals.
External controls of clinical trials are usually done by a drug administration body within a state. Independent oversight requires the review of proposed clinical research projects by qualified individuals independent from the investigators and sponsors.
Independent review boards approve and provide oversight to studies involving human beings. In the US, these are known as Institutional Review Boards (IRBs). In other parts of the world, they are called Independent Ethics Committees (IECs). These boards are composed of researchers, ethicists, legal experts, and community members.
1976 vaccine fiasco (US)
1976 US VACCINE FIASCO
In 1976, an outbreak of the swine flu, influenza A virus subtype H1N1 at Fort Dix, New Jersey caused one death, hospitalized 13, and led to a mass immunisation programme. US President Gerald Ford also received the vaccine for the swine flu. After the program began, the vaccine was associated with an increase in reports of Guillain-Barré Syndrome, which can cause paralysis, respiratory arrest, and death. The immunisation programme was ended after approximately 25% of the population of the United States had been administered the vaccine. Richard Krause, director of the National Institute of Allergy and Infectious Diseases from 1975 to 1984, writes that the government response to the swine flu outbreak was considered to be "too fast".
What are the rights of participants in a clinical trial?
Participants are entitled to a clinical trial that adheres to all legal and ethical standards. In addition, participants have a right to a clear, transparent Informed Consent process before they agree to join the trial.
The "informed consent" process aims at answering any and all questions that might be relevant to a participant’s decision to agree or decline to join a trial.
Only participants who, after having all their questions answered, sign an Informed Consent form can enter the trial.
An Informed Consent document is not a contract. Therefore, a participant may change his or her decision – any participant has the right to withdraw at any point of the trial.
How is the privacy of participants protected?
If a patient agrees to join a trial, some people will need to be told about the participation. Disclosures must adhere to the following protocals:
For participants, what does the end of a clinical trial mean?
The records will be kept for a certain period of time — 15 and more years — as per Good Clinical Practice (GCP) regulations.
The end of a clinical trial has no impact on the confidentiality of those records; they will still be protected from disclosure to third parties.
Many drug companies work with qualified contract research organizations (CROs) and other types of contractors as needed. GCP also requires external organisations to be audited regularly to assure compliance with all policies, procedures and standards.
How are sites selected for clinical trials?
Clinical trials are normally conducted at multiple investigational sites and in many different countries that have the appropriate infrastructure, facilities and training for healthcare professionals and patient availability. Example of COVID-19 trial sites
Diversity: Vaccine trial on up to 30,000 people
The US is set to open the largest trials — 30,000 people to test a government-sponsored jab from this month (July 2020). This will be followed in August with another 30,000 expected to test a British one.
Geographic diversity of trials sites helps ensure that participants are representative of those who need and will benefit from the new medicine, explained Dr. Anthony Fauci, director of the US National Institutes of Health.
Sign up for the Daily Briefing
Get the latest news and updates straight to your inbox
Network Links
GN StoreDownload our app
© Al Nisr Publishing LLC 2026. All rights reserved.