Moderna says COVID-19 vaccine 94.5% effective
Vaccine maker announced interim results on Monday
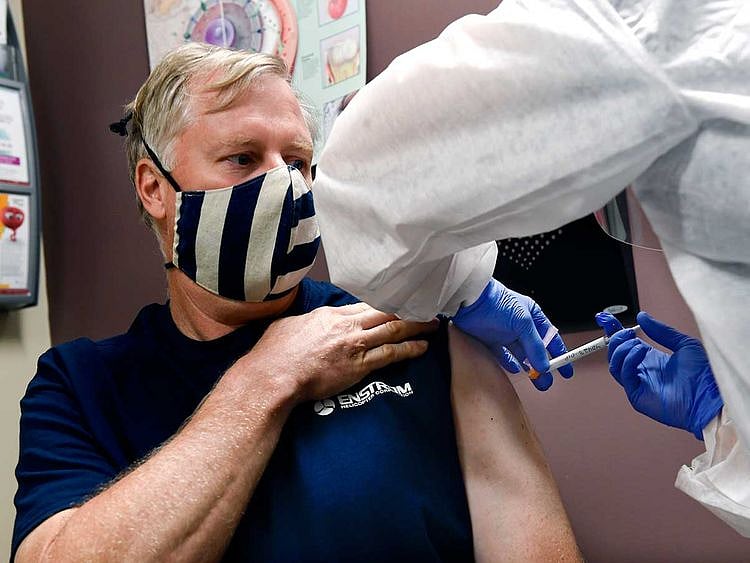
Moderna has announced on Monday that its COVID-19 vaccine is "94.5% effective".
The US pharmaceutical company said its shot provides strong protection against the coronavirus. The company said early data suggests its COVID-19 vaccine is effective, putting a second shot closer to seeking US approval.
On November 10, 2020, Pfizer/BioNTech announced their vaccine is "more than 90%" effective.
"This positive interim analysis from our Phase 3 study has given us the first clinical validation that our vaccine can prevent COVID-19 disease, including severe disease," said Stephane Bancel, Moderna's CEO. Both Moderna and Pfizer/BioNTech shots are based on the messenger RNA technology, a new vaccine development platform.
The results come hot on the heels of similar results from Pfizer, and add to growing confidence that vaccines can help end the pandemic.
Both companies used a highly innovative and experimental approach to designing their vaccines.
Moderna says it is a "great day" and they plan to apply for approval to use the vaccine in the next few weeks.
However, this is still early data and key questions remain unanswered.
How good is it?
The trial involved 30,000 people in the US with half being given two doses of the vaccine, four weeks apart. The rest had dummy injections.
The analysis was based on the first 95 to develop COVID-19 symptoms. Only five of the coronavirus cases were in people given the vaccine, 90 were in those given the dummy treatment. The company says the vaccine is protecting 94.5%.
The data also shows there were 11 cases of severe Covid in the trial, but none happened in people who were immunised. "The overall effectiveness has been remarkable... it's a great day," Tal Zaks, the chief medical officer at Moderna, told BBC News.
What don't we know?
We still do not know how long immunity will last as volunteers will have to be followed for much longer before that can be answered.
There are hints it offers some protection in older age groups, who are most at risk of dying from COVID-19, but there is not full data. Zaks said their data so far suggests the vaccine "does not appear to lose its potency" with age.
And it is not known whether the vaccine just stops people becoming severely ill, or if it stops them spreading the virus too.
All these questions will affect how a coronavirus vaccine is used.
Is it safe?
No significant safety concerns have been reported, but nothing, including paracetamol, is 100% safe.
Short lived fatigue, headache and pain were reported after the injection in some patients.
"These effects are what we would expect with a vaccine that is working and inducing a good immune response," said Prof Peter Openshaw, from Imperial College London.
Prof Trudie Lang, Director, The Global Health Network, Nuffield Department Of Medicine, University Of Oxford: ‘It is very good news indeed to see another vaccine coming through with similar efficacy results as were reported last week from Pfizer. This is also an interim analysis, which means that there were enough cases within the vaccinated volunteers to give statistical significance and allow the team to break the blind to determine who had the active vaccine and who had [a] placebo.’
Stephen Evans, Professor Of Pharmacoepidemiology, London School Of Hygiene & Tropical Medicine: "This announcement from Moderna is a further encouragement that vaccines will be found to not only have an acceptable efficacy, but an efficacy that is much greater than we had anticipated. This press release is more specific than that of others, in that it confirms the numbers in each group, which was able to be guessed at but to have it confirmed is helpful.
"Although they reported efficacy being over 94%, there is statistical uncertainty in this; but based on these data, the likely efficacy will be better than 85% which would be greater than most scientists would have expected.
Severe cases
"This is the first study to report on severe cases and, while uncertainty remains, the finding of no severe cases with the vaccine and 11 cases with placebo is very strong evidence that the vaccine prevents severe as well as mild disease. It is likely that convincing evidence in respect to deaths will probably only be obtained when the vaccine is in use.
"A wide range of people with illnesses and from minority groups were included in the trials as well as substantial numbers of older patients. We will need much more data and a full report or publication to see if the benefit is consistent across all groups, notably the elderly, but this is definitely encouraging progress." PETER OPENSHAW, Professor Of Experimental Medicine At Imperial College London: "This news from Moderna is tremendously exciting and considerably boosts optimism that we will have a choice of good vaccines in the next few months." "This latest press release is based on a study of 30,000 U.S. adults, including many high-risk or elderly persons. This gives us confidence that the results are relevant in the people who are most at risk of COVID-19 and in most need of the vaccines."
Ease of vaccine delivery?
"Moderna have also announced that the vaccine can be kept in a conventional freezer (-20 degrees Celsius) for up to 6 months, and that once thawed the vaccine can be kept for up to 30 days at standard refrigerator (2 to 8 degrees centigrade).
This makes the vaccine much easier to deliver.
"In terms of side effects, news is also quite good. The first dose caused injection site pain in in about 3% of people; the second dose was associated with transient generalized symptoms in about 10% of people with fatigue, muscle aches and flu like symptoms. This seems to indicate that they got the dose about right with acceptable adverse events. These effects are what we would expect with a vaccine that is working and inducing a good immune response." "We need more complete details than we have in this press release, but this announcement adds to the general feeling of optimism about vaccines for Covid-19. What we still don't know is how long any protective immunity may last. For that, we will need to wait."
Network Links
GN StoreDownload our app
© Al Nisr Publishing LLC 2026. All rights reserved.