COVID-19: Do we have a coronavirus cure yet?
A couple of antibody-based drugs have shown promise, but more trials are required
Have we found a cure for COVID-19? No, not yet. There’s also no cure for common flu and viral fever, both of which have been with us for a long time. Flu and viral fever are still treated symptomatically, although some doctors prescribe antiviral drugs to reduce the severity and duration of the symptoms.
If common flu and viral fever do not have a cure, why are we obsessed with finding coronavirus treatments or vaccines. It’s the economy, stupid. Lockdowns and border closures have battered our economies so badly that job losses threaten starvation and hunger in many parts of the world.
Actually, SARS-CoV-2, the virus which causes COVID-19, is not lethal. More than 95 per cent cases tend to be mild, and most patients recover in a fortnight with adequate rest and hydration. But it can be deadly for people with comorbidities (conditions that compromise immunity). So if you have a heart condition or undergone chemotherapy, there’s reason enough to be worried.
Researchers around the world are racing to find a cure or a vaccine, simply because the virus had infected around five million people in less than five months besides killing more than 300,000. What’s more worrying is the astounding pace at which the infections have been travelling. Which is why we have to ramp up our hygiene levels by frequently washing hands, practising social distancing to avoid catching the virus and taking measures to boost immunity.
We have to do it diligently. For there’s no cure yet. Every time there’s news of a prospective cure, our hopes rise. But that optimism is often snuffed out cruelly by the results from the trials.
Remdesivir, hydroxychloroquine and azithromycin have been used with varying degrees of success. Of late, two antibody-based drugs — one from the United States and another from China — have shown plenty of promise. Then there’s the miracle cure from Madagascar. Bangladesh doctors too offered a ray of hope, saying they have had success in treating COVID-19 with a cocktail of drugs. (Please note that most drugs discussed here are experimental and should be administered only under the supervision of qualified medical personnel).
The antibody shield from America
A US-based company says that its therapeutic antibody candidate can block the COVID-19 infection.
The company Sorrento Therapeutics is buoyed by reports from preclinical studies which showed that its STI-1499 could fully inhibit the infection of healthy cells at a significantly low dose. Given its high potency, Sorrento plans to develop it as a monotherapy, COVI-GUARD, Pharmaceutical Technology magazine reported
Since STI-1499 was found to be a potentially strong antibody drug candidate, the company aims to use it as the first antibody in the antibody cocktail, COVI-SHIELD.
What’s the new Chinese drug?
The Chinese drug, which is believed to be a potential cure, is a neutralised antibody (produced by the human immune system to fight diseases) isolated from the blood of recovered patients.
The drug has been successful at the animal testing stage, and after clinical trials, it is expected to be ready for use later in the year.
Developed by the Peking University in China, the drug is also said to shorten the recovery time of COVID-19 patients besides offering short-term immunity, according to a study on the research published in the journal Cell.
“When we injected neutralising antibodies into infected mice, after five days the viral load was reduced by a factor of 2,500,” Sunney Xie, director of the university’s Beijing Advanced Innovation Centre for Genomics, told AFP. “The hope is these neutralised antibodies can become a specialised drug that would stop the pandemic.”
The miracle cure from Madagascar
Madagascar feels it has the medicine to eliminate coronavirus. It’s a herbal drink extracted from the plant Artemisia annua. The Madagascar government claims to have put the herbal remedy, called “COVID-Organics, through three weeks of testing on less than 20 people, according to a BBC report.
Madagascar President Andry Rajoelina calls the herbal tonic a “miracle cure” for COVID-19. “The patients who have healed have taken no other product than COVID-Organics,” Rajoelina told France 24. “The patients tend to heal [in] seven to 10 days.”
Previously used for the treatment of malaria, the herbal drink is being distributed for free to the poor and vulnerable and to schools in in the African country.
More than 20 African countries have placed orders for “COVID-Organics,” but it certainly has to undergo more trials to test its efficacy against the virus.
One of the significant tests is being carried out by Germany’s Max Planck Institute of Colloids and Interfaces, which is collaborating with the US company ArtemiLife to determine the plant extract’s effectiveness against the coronavirus.
Scientists in several African countries are conducting tests as well. The World Health Organisation too has shown interest in studying the effectiveness of the herbal tea.
The success story from Bangladesh
Several countries have had success in treating coronavirus using a cocktail of antiviral drugs. But none of them has turned into a viable cure.
Bangladesh doctors are excited at the results of administering a mixture of two widely available drugs. The medical team used antiprotozoal medicine Ivermectin in a single dose with the Doxycycline to cure patients with acute COVID-19 symptoms.
“We have got astounding results. Out of 60 COVID-19 patients, all recovered as the combination of the two drugs were applied,” Dr Mohammed Tarek Alam, head of medicine department at private Bangladesh Medical College Hospital told the Press Trust of India.
Trump trusts in hydroxychloroquine
US President Donald Trump says he’s been taking hydroxychloroquine for more than a week despite warnings about its potential side effects.
“You’d be surprised at how many people are taking it, especially the frontline workers before you catch it, the frontline workers, many, many are taking it. I happen to be taking it,” the 73-year-old told reporters at the White House.
Hydroxychloroquine has been used to treat autoimmune diseases like lupus and rheumatoid arthritis. The US Food and Drug Administration has authorised for its use in only some cases.
Referring to reports of heart problems in COVID-19 patients, the FDA issued an advisory cautioning against the use of the drug outside hospitals. The agency warned that hydroxychloroquine is not safe and effective.
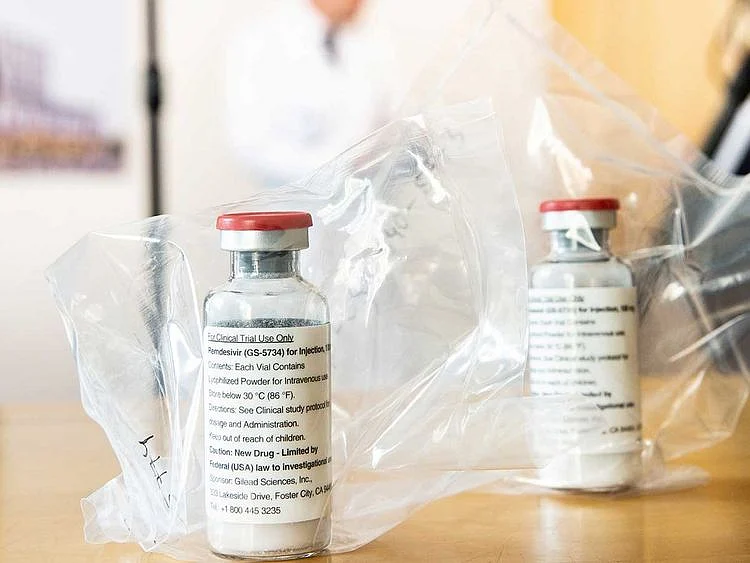
Why Remdesivir is still in the news
Remdesivir is an experimental broad-spectrum antiviral drug developed to fight Ebola. It has been found to be effective in fighting the coronavirus. The treatment is not yet approved for humans, but several trials are underway.
Last month, Stat, the medical news website, reported that Phase 3 clinical trials at the University of Chicago showed remdesivir’s effectiveness in treating COVID-19 patients.
Gilead Sciences, the US biotech giant who makes remdesivir, has ramped up the production of the drug after the US issued an emergency use authorisation as it helped patients recover faster.
The race for COVID-19 therapy
Governments and private agencies around the world are pouring in millions of dollars in the search for a cure. With economies around the world taking a colossal battering, the need for finding a viable treatment or vaccine has never been more urgent.
Jobs are vanishing, and livelihoods are under threat. Starvation and hunger will follow. The world can’t let that happen. A drug has to be found, for the sake of humanity.
Sign up for the Daily Briefing
Get the latest news and updates straight to your inbox
Network Links
GN StoreDownload our app
© Al Nisr Publishing LLC 2026. All rights reserved.