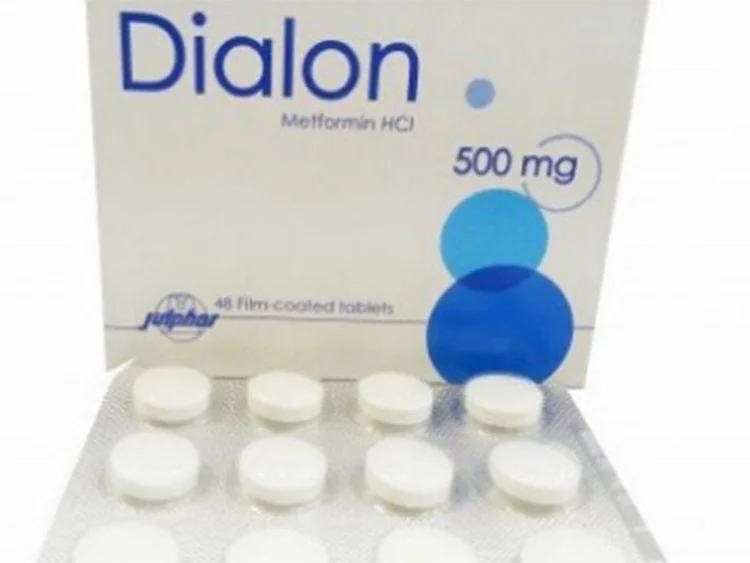
Dubai: The Ministry of Health and Prevention issued a circular on Monday withdrawing a diabetic medicine from the UAE market.
The Dialon 500mg tablets contained the active ingredient metformin, which was manufactured by the pharmaceutical company Julphar.
The circular stated that the generic medicine was withdrawn after observing the tablet’s poor performance during comparative studies of the branded product.
According to the circular, the ministry also received another health and safety report from the Saudi Food and Drug Administration indicating the failure of the product.
Diabetes is a chronic disease with the potential of affecting all organs and organ systems. About 15 per cent of Dubai's population are diabetic. Gulf News archives
Dr Amin Hussain Al Amiri, Assistant Under-Secretary for Public Health Policy and Licensing at the ministry, said the circular to withdraw the medicine was issued to directors of medical zones, government and private hospital directors, doctors, pharmacists and pharmacy assistants to the directors of state and private pharmacies.
Al Amiri said that the Ministry of Health and Prevention is in daily contact with local pharmaceutical factories, the US Food and Drug Administration, the European and Australian Medicines.
“If any warning is issued, a circular is immediately issued to all health authorities to remove these drugs and destroy them,” Al Amiri was quoted by the state news agency WAM.
He appealed to members of the society not to circulate or use the product, urging the public to report any side effects by contacting the ministry on 04-3201448 or by e-mail at pv@moh.gov.ae.
Sign up for the Daily Briefing
Get the latest news and updates straight to your inbox
Network Links
GN StoreDownload our app
© Al Nisr Publishing LLC 2026. All rights reserved.