Needle-free, nasal COVID-19 vaccine more effective?
Big shift coming: SARS-CoV-2 vaccines set to move from human trials to manufacturing
Also In This Package
US: Volunteers flock to vaccine trials in Florida
$4 to $145: How much will the COVID-19 vaccine cost?
Sputnik-V vaccine, coronavirus and the Libyan crisis
India's COVID vaccine price: 'Less than water bottle'
WHO wants to review Russian COVID-19 vaccine data
Latest COVID-19 vaccine deals: All you need to know
Vaccine updates: Finish line in sight?
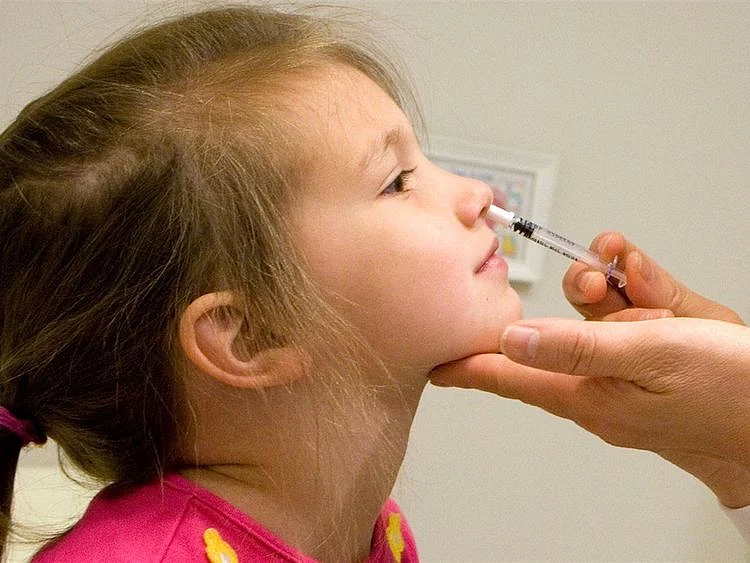
DUBAI: Vaccines given through the nose similar to the flu shot invented by an Arab-American scientist, could work better than intramuscular jabs in preventing the spread of the coronavirus pandemic, according to a new research.
That, at least, is the hope of a research team who did their comparative study in mice, which showed nasal vaccines were more effective in conferring immunity to SARS-CoV-2 than muscle shots.
Now that laboratory, animal trials are done and human trials of coronavirus vaccines are in advanced stages, the game will soon shift to mass manufacturing.
A new question arises: What is the best way to deliver such vaccines?
The study, published in the journal Cell as a pre-proof, has shown that an intranasal administration of an experimental COVID-19 vaccine worked better in animal models, than the intramuscular route.
Based on available literature, most COVID-19 vaccines in development are planned to be intramuscular shots.
Vaccine administration routes include:
In the new study, the researchers found that the nasal delivery created a strong immune response throughout the body. But it was found particularly effective in the nose and respiratory tract, preventing the infection from taking hold in the body.
Vaccine delivered via the nose, often the initial site of infection, is nothing new. It is being used in the flu shots by the millions each year. Spritzing a vaccine is also deemed less traumatic to children.
What’s the study about and who was behind it?
The research evaluated the protective activity in mice of a vaccine encoding the so-called "spike" protein in challenge studies with SARS-CoV-2 and mice expressing the human angiotensin-converting enzyme (ACE) 2 receptor.
The 31-member team of scientists was led by Ahmed Hassan of the Washington University (WU) School of Medicine, at St. Louis, Missouri. Hassan was joined from colleagues from The Andrew M. and Jane M. Bursky Center for Human Immunology & Immunotherapy Programs (at WU), the Center for Infectious Disease and Vaccine Research, the La Jolla Institute for Immunology and the Department of Epidemiology at the University of North Carolina at Chapel Hill.
What were their findings?
In animal subjects (mice), the scientists demonstrated that a nasal shot does offer immunity from SARS-CoV-2, the virus that causes COVID-19.
Interestingly enough, the team’s findings suggest just one dose is enough to do the trick.
The researchers stated: “A single-dose intranasal ChAd vaccine protects upper and lower respiratory tracts against the new viral infection in mice susceptible to the novel coronavirus.”
What vaccine platform did the researchers use?
The researchers used a chimpanzee adenovirus-vectored vaccine to evaluate the protective potential of their nasal shot.
The vaccine encoded a “pre-fusion” stabilised spike protein (ChAd-SARS-CoV-2-S) in challenge studies with SARS-CoV-2. The mice were tweaked to express the human angiotensin-converting enzyme (ACE) 2 receptor.
Did the study results show the nasal vaccine worked better?
Yes. Two groups of mice were immunised – one via a mist, and another intra via an intramuscular shot.
After the results came, the scientists stated: “Intramuscular dosing of ChAd-SARS-CoV-2-S induces robust systemic humoral and cell-mediated immune responses and protects against lung infection, inflammation, and pathology but does not confer sterilising immunity, as evidenced by detection of viral RNA and induction of anti-nucleoprotein antibodies after SARS-CoV-2 challenge.
Here’s the interesting part: “In contrast, a single intranasal dose of ChAd-SARS-CoV-2-S induces high levels of neutralising antibodies, promotes systemic and mucosal IgA and T cell responses, and virtually completely prevents SARS-CoV-2 infection in both the upper and lower respiratory tracts.”
Therefore, they stated, “intranasal administration of ChAd-SARS-CoV-2-S is a candidate for preventing SARS-CoV-2 infection and transmission, and curtailing pandemic spread.”
After this nasal vaccine study in mice, what's next?
The study authors hope to test the vaccine in non-human primates in the near future, before going into human trials.
Which vaccine manufacturer plans to take the nasal route?
At least one vaccine maker has publicly declared their plan to do the mist vaccine.
A top official of India’s Bharat Biotech has expressed their intent to introduce a nasal vaccine for COVID-19, partly to make it easier on children.
Suchitra Ella, director of India’s Bharat Biotech, told the Indian media that they are working on a coronavirus vaccine based on the seasonal flu nasal mist platform.
The Hyderabad-based Bharat Biotech, one of the world's top vaccine makers, has commercialised 16 vaccines, including one for H1N1 (swine flu), Rotavirus, Hepatitis-B, Typhoid, Polio, Diphtheria.
For coronavirus, it is currently developing and testing the shot — called “Coroflu” — in collaboration with virologists at the University of Wisconsin-Madison and the vaccine company FluGen.
Covaxin, also developed by Bharat, is India's first "indigenous" SARS-CoV-2 vaccine. It's not immediately clear which one of the two (Coroflu or Covaxin) would be eventually developed as a mist-type vaccine.
What other vaccines are given through intranasal administration?
The nasal flu vaccine is a classic example of a nasal shot.
Ella said: “Vaccines can be delivered in different forms…There are many routes. The polio vaccine, for example, is an oral drop. But this time around, the route that we want to take is the nasal route. We are working towards getting that delivery model.”
“This (nasal coronavirus vaccine) can be handled and it has been done in the past with the flu, which is a classic example. We are pretty hopeful that we would be able to deploy a similar route, or take a similar path when it comes to the COVID-19 as well,” Ella added.
The flu (influenza) is a virus which comes around every year. The virus is known to mutate. At the beginning of each flu season (typically from October through May), health authorities advise the medical fraternity of the flu virus strain likely to become dominant during that period, and the vaccine to be given to the population.
FLU DEATHS
The US CDC estimates that influenza has resulted in between 9 million to 45 million illnesses, between 140,000 to 810,000 hospitalisations and between 12,000 to 61,000 deaths annually since 2010. In the UK, childhood deaths from influenza A (H1N1) is estimated at 2 per 1 million for under 14s.
(Source: The Lancet)
When was the first nasal flu vaccine approved?
In 2003, the US Food and Drug Administration approved "FluMist", a nasal spray flu vaccine, following 40 years of research by University of Michigan (UM) professor Hunein “John” Maassab.
FluMist, approved for use by healthy people ages 5-49, is a cold-adapted, "live-attenuated" (virus is still alive, but weakened), trivalent influenza virus vaccine. It is the first flu vaccine delivered as a nasal mist to be commercially available in the United States. Other vaccine makers soon followed the nasal route.
With the nasal spray flu vaccine, who can be vaccinated?
The CDC guidelines approve its use in healthy non-pregnant individuals, 2 years through 49 years old. People with certain medical conditions, however, should not receive the nasal spray flu vaccine, according to CDC guidelines.
What flu viruses does the nasal spray vaccine protect against?
For the 2020-2021 flu season, all nasal spray influenza (flu) vaccines are "quadrivalent". It means they will be made using four flu viruses: an influenza A(H1N1) virus, an influenza A(H3N2) virus and two influenza B viruses, the CDC said.
How many nasal spray vaccines are used each year?
In 2009, the CDC rolled out the first H1N1 (swine flu) vaccines in US in the form of a nasal spray, initially with 3.4 million doses of MedImmune (AstraZeneca). It was later bumped up to about 20 million doses a week. It was given to people in America for free. That year alone, the US has ordered 195 million doses of H1N1 swine flu vaccine from five pharmaceutical companies.
Sign up for the Daily Briefing
Get the latest news and updates straight to your inbox
Network Links
GN StoreDownload our app
© Al Nisr Publishing LLC 2026. All rights reserved.