COVID-19: Sinopharm vaccine booster needed six months after second dose in UAE, says NCEMA
UAE adopts proactive strategy to provide maximum protection against COVID-19
Also In This Package
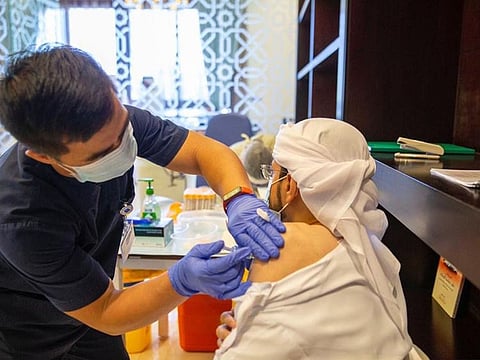
Abu Dhabi: As part of the UAE’s proactive strategy to provide maximum protection to residents, an additional supportive dose of Sinopharm will now be available for people who have received the vaccine previously and have completed more than six months since the second dose, the National Emergency Crisis and Disasters Management Authority (NCEMA) said on Tuesday.
The priority to receive an additional dose of Sinopharm is for senior citizens and people with chronic diseases, the authority said.
It said relevant national committees are constantly keeping pace with global updates regarding vaccine development and they are being evaluated periodically.
NCEMA said vaccines are approved for full use or emergency use, according to regulations adopted in the country after evaluating their safety and effectiveness.
Vaccines for children
It said the Ministry of Health has approved the emergency use of the COVID-19 Pfizer-Biontech vaccine for those aged between 12 and 15 years. This was based on the results of clinical studies, the strict evaluation for emergency use permit and local assessment in keeping with approved regulations.
Expanding the scope of vaccination to include this group would open the door for vaccinating a large number of residents in the country, and vaccinating the largest segment of society, thus contributing to reaching acquiring immunity.
"Despite the low number of positive cases among children, vaccination is very important due to the return of students to schools next year. Our message to parents is to trust that vaccination will help all of us feel safe and maintain the health and safety of our children."
"Child vaccination is a fundamental issue, as it will relieve the burden on parents whose children are still pursuing remote learning. Therefore, we call upon parents to take the initiative to vaccinate their children in order to ensure their health and safety," it added.
When did UAE approve Sinopharm?
It was on December 9, 2020 that the UAE officially registered the inactivated vaccine developed by Sinopharm CNBG’s Beijing Institute of Biological Products.
As reported by Gulf News earlier, Sinopharm CNBG had announced last July that the COVID-19 vaccine will be tested in the 4 Humanity trials which had seen a positive response in the earlier stages of testing. All volunteers in China who had participated in Phase I and Phase II trials had successfully generated antibodies against COVID-19 when two doses of the vaccine were delivered 28 days apart. In addition, no adverse reactions had been recorded.
Following an online video conference between Abu Dhabi and Beijing, UAE health authorities announced the commencement of the world’s first phase III clinical trials of a COVID-19 inactivated vaccine on June 23. In attendance were UAE Health Minister, Abdulrahman Al Owais and Abdullah Al Hamed, chairman of Abu Dhabi’s health regulator, the Department of Health (DoH).
On July 16, two senior health officials in Abu Dhabi — Al Hamed, and DoH undersecretary Dr Jamal Al Kaabi — became the first people to receive the vaccine shots for the 4 Humanity trials in the UAE.
Sign up for the Daily Briefing
Get the latest news and updates straight to your inbox









