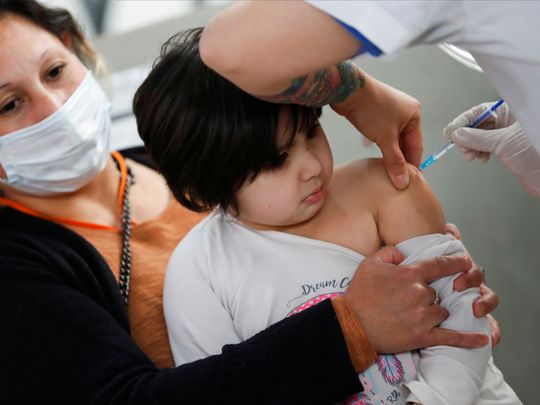
Dubai: Bahrain has approved the Sinopharm COVID-19 vaccine for children aged between 3 and 11 years from October 27, the state media office said on Tuesday.
The Pfizer COVID-19 vaccine would soon be approved for children between 5 and 11, it also said.
The national medical team also stressed the importance of vaccinating the targeted age group “to protect them and their families and society, especially in light of the long incubation period of the virus in children in the event of infection.”
Those interested can register for the vaccination through the Ministry of Health website: healthalert .gov.bh or through the dedicated mobile application.
The UAE in August rolled out Sinopharm vaccine to children aged 3-17. But the UAE Health Ministry said that the vaccination for the age group from 3-15 years is optional, not mandatory, adding that the vaccine approved for children aged three to 11 years is Sinopharm, while children aged 12 to 15 years can take either Sinopharm or Pfizer-BioNTech.
Jordan in July begun vaccinating children aged 12 years and older against COVID-19.








