If you use a phone, thank these 3 lithium-ion battery pioneers
Lithium-ion batteries first came out in 1991...what happened before and next
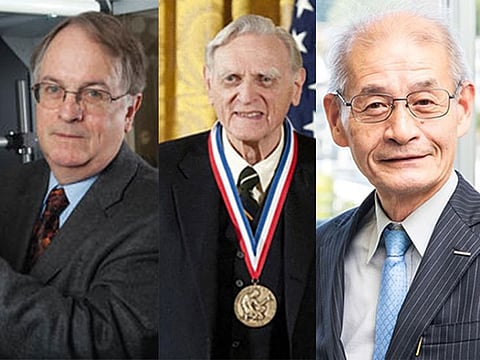
Dubai: We take it everywhere we go. If you have any sort of phone, then you have it — or a version of it.
Lithium-ion batteries were first launched in 1991. Until about 20 years ago, they were still quite rare, competing with other battery types.
Today, lithium-ion batteries are undisputed winners, ubiquitous and unobtrusive — in your ears, on your wrist, phone and even your heart (pacemaker implants).
This lightweight, rechargeable and powerful battery used in everything from mobile phones to laptops and electric vehicles, is changing the world.
On Wednesday, three scientists who contributed to the development of lithium-ion batteries — Stanley Whittingham of Britain, John B. Goodenough of the US, and Akira Yoshino of Japan — won the Nobel Prize in Chemistry 2019.
TIMELINE:
Since then, lithium-ion batteries have brought the greatest benefit to humankind.
They allowed for the development of laptop computers, mobile phones, electric vehicles as well as small and large-scale storage of energy generated by solar and wind power.
Here's a quick profile of the "lithium brothers" and their part in this awesome invention.
1. Stanley Whittingham
Stanley Whittingham, a British-American chemist, worked to develop methods to help lead the world towards fossil fuel-free energy technologies.
His research on superconductors led him to the discovery of an extremely energy-rich material, which he used to create an innovative cathode in a lithium battery.
The cathode was made of titanium disulphide which, at a molecular level, has spaces that can house — intercalate — lithium ions.
CATHODE
From the Greek word "kata" (down), and "nodos" (way): The negatively-charged electrode by which electrons enter an electrical device.
The battery’s anode was partially made from metallic lithium, known for its strong drive to release electrons. This resulted in a battery that literally had great potential, at just over two volts.
ANODE
From the Greek word "ana" (up) and "nodos" (way): The positively-charged electrode by which the electrons leave an electrical device, such as a primary cell, that supplies current.
However, metallic lithium is reactive and the battery was too explosive to be viable.
Whittingham is currently a professor of chemistry and director of both the Institute for Materials Research and the Materials Science and Engineering program at Binghamton University, part of the State University of New York (SUNY).
LITHIUM
Lithium is an an ancient element. Believed to have been created during the first minutes of the Big Bang, lithium is the lightest solid element. Lithium’s main weakness – its reactivity – is also its strength. It is perfect for powering mobile phones and electric vehicles. Mankind first became aware of it in 1817, when Swedish chemists Johan August Arfwedson and Jöns Jacob Berzelius purified it out of a mineral sample from Utö Mine, in the Stockholm archipelago. Berzelius named the new element after the Greek word for stone, "lithos". The Swedish chemists did not actually find pure metallic lithium, but lithium ions in the form of a salt.
2. John B. Goodenough
Prof. John Goodenough, working at the University of Texas at Austin, USA, predicted that the cathode would have even greater potential if it was made using a metal oxide instead of a metal sulphide.
He knew about Whittingham’s revolutionary battery. But his specialised knowledge of the interior of matter told him that its cathode could have a higher potential — if it was built using a metal oxide instead of a metal sulphide.
A few people in his research group were then tasked with finding a metal oxide that produced a high voltage when it intercalated lithium ions, but which did not collapse when the ions were removed.
After a systematic search, in 1980 he hit a breakthrough.
Whittingham’s battery generated more than 2 volts. Goodenough's research doubled this, by using lithium cobalt oxide in the cathode (cobalt oxide intercalated with lithium ions) — producing as much as four volts.
The use of cobalt oxide in the lithium battery’s cathode was successful beyond what Goodenough had dared to hope. This was an important breakthrough and led to much more powerful batteries.
Another key to his success: The knowledge that batteries did not have to be manufactured in their charged state, as done previously. Instead, they could be charged afterwards.
In 1980, Dr Goodenough hit a breakthrough, and published his discovery of a new, energy-dense cathode material which led to batteries with lower weight, more power and higher capacity.
This was the main driver of the wireless electronics revolution today.
Today, at 97, Dr Goodenough continues to work on batteries, as a professor emiritus at the University of Texas at Austin, USA, along with other researchers.
3. Akira Yoshino
In 1985, with Goodenough’s cathode as a basis, Dr Akira Yoshino of Meijo University in Nagoya, Japan, created the first commercially viable lithium-ion battery through Asahi Kasei Corp.
He knew of Goodenough’s technique, and used lithium-cobalt oxide as the cathode. Dr Yoshino tried using various carbon-based materials as the anode. The result: A functional and commercial mass-market rechargeable lithium-ion battery.
INTERCALATION
In chemistry, "intercalation" is the reversible inclusion or insertion of a molecule (or ion) into materials with layered structures, such as graphite (a crystalline form of carbon with its atoms arranged in a hexagonal structure).
In the past, researchers had shown that lithium ions could be "intercalated" (inserted) in the molecular layers in graphite, but the graphite was broken down by the battery’s electrolyte.
Prof. Yoshino’s breakthrough came when he used petroleum coke, a carbon material and by-product of the oil industry. The petroleum coke, like the cathode’s cobalt oxide, can intercalate lithium ions.
When he charged the petroleum coke with electrons, the lithium ions were drawn into the material.
When he turned on the battery, the electrons and lithium ions flowed towards the cobalt oxide in the cathode. This posed a much higher potential.
Yoshino's petroleum coke, like the cathode’s cobalt oxide, can intercalate lithium ions.
The result was a stable, lightweight, hardwearing battery with a high capacity and produces a remarkable 4 Volts. Even more, it could be charged hundreds of times before its performance deteriorated.
The key advantage of lithium-ion batteries today: they are not based upon chemical reactions that break down the electrodes, but upon lithium ions flowing back and forth between the anode and cathode.
Since they first entered the market in 1991, lithium-ion batteries have revolutionised the mobile electronics. They have laid the foundation of a wireless society, unleashing a revolution in communications and mobility.
USES, FORMS
Small lithium batteries are commonly used in small, portable electronic devices, lick PDAs, watches, digital cameras, thermometers, calculators, personal computer BIOS, communication equipment and remote car locks.
They are available in many shapes and sizes, with a common variety being the 3 volt "coin" type manganese variety, typically 20 mm in diameter and 1.6–4 mm thick.
There are numerous formats of Li-ion batteries, and various techniques to cool them. In 2017, Panasonic started producing a new, the 2170 battery cell format for Tesla’s new Model 3 at Gigafactory 1 in Nevada.
After lithium-ion batteries, what's next?
Lithium-ion batteries have become a standard for powering personal electronics, mobile devices and EVs. But they can explode. When improperly designed, they're dangerous for use in aircraft.
Some phone models and even laptop models had been banned from airports due to exploding batteries and fire hazards.
Worse, the commercial lithium-ion batteries have put a strain on the world's supply of cobalt and nickel — two metals integral to current battery designs — and sent prices surging.
As of October 8, official prices per tonne stood at $37,085 for cobalt and $17,560 for nickel on the London Metal Exchange (LME).
Safer, cheaper, more powerful
Researchers continue to find ways to make lithium-ion batteries safer, cheaper and more powerful. Currently, there several developments in lithium-ion battery research.
In a bid to develop cheaper, safer lithium-based batteries that rely less on those expensive and scarce metals, researchers at the Georgia Institute of Technology have developed a promising new cathode and electrolyte system.
Using lower-cost transition metal flourides and a solid polymer electrolyte to replace expensive cobalt/nickel and traditional liquid electrolyte makes more sense.
Researchers in the US have been developing a promising new technique.
One involves the use of cathode and electrolyte system that replaces expensive metals (cobalt and nickel) and traditional liquid electrolyte with lower-cost transition metal fluorides and a solid polymer electrolyte.
IRON FLOURIDES
In a study published September 9, 2019 in the journal Nature Materials and sponsored by the Army Research Office, a research team from Georgia Institute of Technology fabricated a new type of cathode from iron fluoride active material; alongside solid polymer electrolyte nanocomposite.
Iron fluorides offer key advantage: They have more than double the lithium capacity of traditional cobalt- or nickel-based cathodes. More importantly, iron is 83 times cheaper than cobalt and 40 times cheaper than nickel.
Steel, a highly abundant metal found in many places around the world, stood at $446 per tonne on LME on October 8, 2018.
The polymer-based electrolyte offer two key advantages:
BREAKTHROUGH IN BATTERY RESEARCH
Engineers at the University of California San Diego have developed a breakthrough in electrolyte chemistry that enables lithium batteries to run at temperatures as low as -60 °C with excellent performance.
The new electrolytes also enable electrochemical capacitors to run as low as -80 °C — their current low-temperature limit is -40 °C. While the technology enables extreme low-temperature operation, high performance at room temperature is still maintained.
According to reports, the new electrolyte chemistry could also increase the energy density and improve the safety of lithium batteries and electrochemical capacitors.
In March 2019, researchers at Penn State published in Nature Materials results of their study showing ways to boost power density, performance, and safety of lithium ion batteries using newly-developed, solid-electrolyte interphase (SEI).
As the demand for higher-energy-density lithium metal batteries increases — for electric vehicles, smartphones, and drones — stability of the SEI has been a critical issue. This has curbed their advancement. A salt layer on the surface of the battery's lithium electrode insulates it and conducts lithium ions.
The degradation of the SEI, one of the least-understood components of Li-ion batteries, contributes to the development of dendrites. These are needle-like formations that grow from the lithium electrode of the battery and negatively affect performance, durability and safety.
In the Penn State project, led by chemistry doctoral student Yue Gao, researchers used a polymer composite to create a much better SEI. The researchers claim that reactive polymer also reduces the weight and manufacturing cost. This could make lithium metal batteries last.
Today, there's a crazy amount of research become done on Li-ion battery technology. The aim: Enhance their power and safety, reduce weight and cost.
The foundations of these cool batteries were laid down by the three great pioners — Whittingham, Goodenough and Yoshino.
Sign up for the Daily Briefing
Get the latest news and updates straight to your inbox





