Highlights
- Research data show that up to 80% of laboratory-acquired infections occured via aerosol transmission.
- Thousands of laboratory "escape" incidents had been reported in the past.
- Once an epidemic gets going, the scale of the problem doubles every week.
- Human error plays a big part in laboratory escapes of dangerous pathogens.
It’s entirely possible the world will never know for sure — or find the “smoking gun” — whether the SARS-CoV-2’s origin was from nature (i.e. zoonotic, passed from animal/insect to human) or was a result of a laboratory leak.
Here's one fact: dangerous pathogens sometimes escape the lab, with deadly results. Published data on the “escape” of deadly biological agents do exist. As we look beyond the controversy surrounding the on-going coronavirus pandemic’s origins, we focus on documented leaks of pathogens that caused infections from labs. A look into what happened:
Were there any episodes of accidental lab release of infectious diseases?
Yes. Hundreds of "accidental releases” of virulent pathogens had been documented. The term used in scientific circles for such phenomenon in laboratory-acquired infections (LAIs).
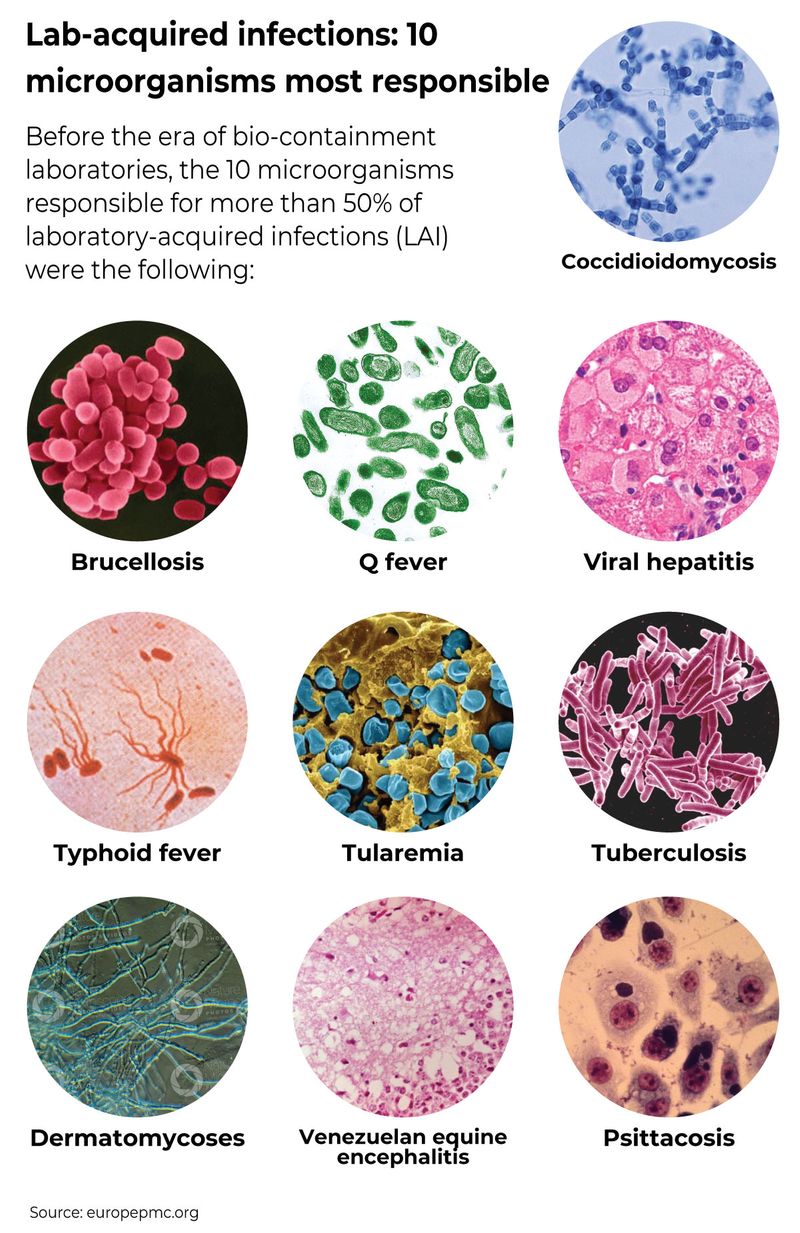
What are 10 microorganisms most responsible for LAIs?
Before the era of bio-containment laboratories, the 10 microorganisms responsible for more than 50% of laboratory-acquired infections (LAI) were the following:
How many laboratory leaks were documented and reported in the past?
Lab-associated infections are as old as laboratories themselves. Here's an overview, based on documented leak events (periods may overlap, depending on sources cited):
1885 to 1915:
The first recorded laboratory-acquired infection (LAI) was a case of typhoid fever in 1885. It was soon followed by cases of brucellosis, tetanus, cholera, diphtheria and sporotrichosis — recorded in the years from 1887 to 1904. The “survey” the first lab-acquired infections was published by Karl Kisskalt in 1915. Between 1885 and 1915, he found 50 cases of typhoid fever, six of which were fatal. [https://bit.ly/2RXwDn7]
1915, 1929 and 1939:
US surveys recorded infections with psittacosis and Q fever that followed the grinding and centrifugation of yolk sac cultures (highlighting importance of the aerosol route).
1951 to 1978:
Available literature shows LAI and associated death rate in the US and Europe during this period. A total of 7,068 infections were reported during this period (1,275 in 1951; 1,073 in 1961; 641 in 1965; 3921 in 1976; and 158 in 1978). There were 288 associated deaths reported during this period. [https://bit.ly/3cJ7gfV]
Dِuring this period, at least 80 cases and 3 deaths were reported as a result of three separate escapes of the smallpox virus from two different accredited smallpox laboratories in the UK, according to a 2014 report written Dr. Martin Furmanski of the Scientist’s Working Group on Chemical and Biologic Weapons under the Center for Arms Control and Nonproliferation.

2004-2010:
A 2017 report by the US CDC states that at least 11 laboratory-acquired infections (LAIs) were recorded for microorganisms listed as “biological select agents and toxins from the period 2004 to 2010. [https://bit.ly/3iFP3DY]
Here’s the breakdown of reported LAI cases in the US based on the CDC report:
- 6 cases: due to Brucella spp. (causes Brucellosis, a bacterial infection, in humans)
- 4 cases due to Francisella tularensis (causes Tularemia or rabbit fever/deer fly fever in humans)
- 1 one case due to Coccidioides immitis/posadasii (cause of valley fever in humans)
[https://bit.ly/3iFtYti]
This report may be deficient. In a 2014 study covering the years 2008 and 2012, Harvard University epidemiologist Dr Marc Lipsitch found that government data on US biosafety labs reveal accidents estimated to be between “100 and 275 potential releases” of pathogens each year in labs that deal with select agents.
275
number of 'potential releases' of pathogens from US biosafety lab recorded each year from 2008 to 2012The reports include “banal accidents” like spills and record-keeping errors, and very few workers were infected. Beyond 2012, there were other lab leak incidents reported:
2014:
Forgotten vials of smallpox were found in a cardboard box in a research centre near Washington. The dangerous live pathogens were accidentally sent to laboratories that were neither expecting them nor equipped to deal with them.
2015:
The US military accidentally shipped live anthrax samples instead of dead spores to as many as nine labs across the country and a military base in South Korea.

What other other lab leaks were reported?
2003 to 2004: There had been six separate “escapes” reported from virology labs studying SARS: one each in Singapore and Taiwan, and in four separate events at the same laboratory in Beijing, according to Dr Furmanski's report.
August 2003:
A 27-year-old virology graduate student at the National University of Singapore was infected by SARS. He had not worked directly with SARS, but SARS was present in the virology laboratory where he worked with West Nile Virus (WNV). Investigation showed that his preparation of WNV was contaminated with SARS virus, and that this was the likely origin of his infection. After falling ill on Aug 26, he sought outpatient medical care in several venues, and was admitted to the hospital only on September 3. He recovered and there were no secondary cases. Investigation revealed multiple shortcomings in infrastructure, training and observed procedures at the laboratory, and remedial actions were ordered.
December 2003:
The second escape was reported in Taiwan in, when a SARS research scientist fell ill on a return airflight after attending a medical meeting in Singapore December 7- 10. Although he felt is illness was SARS, he remained at home for 5 days, unwilling to seek medical care because he dreaded bringing disgrace to himself and his institution. He was only persuaded to enter the hospital when his father threatened to commit suicide.
Preliminary investigation implicated a laboratory exposure due to an attempt to decontaminate a bag of leaking biological waste, suspected to have had no proper protection and against protocol the day before he left for Singapore. His 74 contacts in Singapore were put under quarantine for ten days. Fortunately, none developed SARS.
April 17, 2004:
The investigation at NIV also uncovered an unrelated laboratory infection in a 31-year old male laboratory researcher at the NIV who fell ill on April 17, 2004. The entire NIV institute was closed and all of its 200 employees placed in quarantine in a hotel. Subsequent investigation confirmed these first three cases as SARS, and eventually identified a total of nine cases, in three generations, including health care workers and their family contacts.
April 22, 2004:
China reported a suspected case of SARS in a 20-year-old nurse who fell ill on April 5, 2004 in Beijing. The next day, it reported she had nursed a 26-year-old female laboratory researcher who had fallen ill on March 25, 2004. Still ill, the researcher had traveled by train to her home in Anhui province where she was nursed by her mother, a physician, who fell ill on April 8 and died April 19.
The researcher had worked at the Chinese National Institute of Virology (NIV) in Beijing, part of China’s Center for Disease Control (CDC), and which was a major center of SARS research.
The two primary patients had not worked directly with live SARS virus. WHO investigators had expressed “serious concerns” regarding biosafety procedures at the NIV at that time, according to the WHO Global Alert and Response (GAR) update issued on May 18, 2004.
Was there anything done to improve the situation?
Yes, an expert committee from WHO investigated the laboratories and their procedures, and recommended improvements. On December 18, 2003, WHO released a new protocol for handling SARS specimens, with special emphasis on reducing risk of and performing surveillance to detect laboratory infections.
Although this protocol was clearly created after the first (Singapore) escape, WHO chose to “parse” its words to avoid offending members. It chose to treat the risk as hypothetical.
The WHO wrote in its report then: “The possibility that a SARS outbreak could occur following a laboratory accident is a risk of considerable importance, given the relatively large number of laboratories currently conducting research using the SARS-CoV or retaining specimens from SARS patients. These laboratories currently represent the greatest threat for renewed SARS-CoV transmission through accidental exposure associated with breaches in laboratory biosafety.”
“The possibility that a SARS outbreak could occur following a laboratory accident is a risk of considerable importance, given the relatively large number of laboratories currently conducting research using the SARS-CoV or retaining specimens from SARS patients. These laboratories currently represent the greatest threat for renewed SARS-CoV transmission through accidental exposure associated with breaches in laboratory biosafety.”
[Source: https://bit.ly/3xmnnb5]
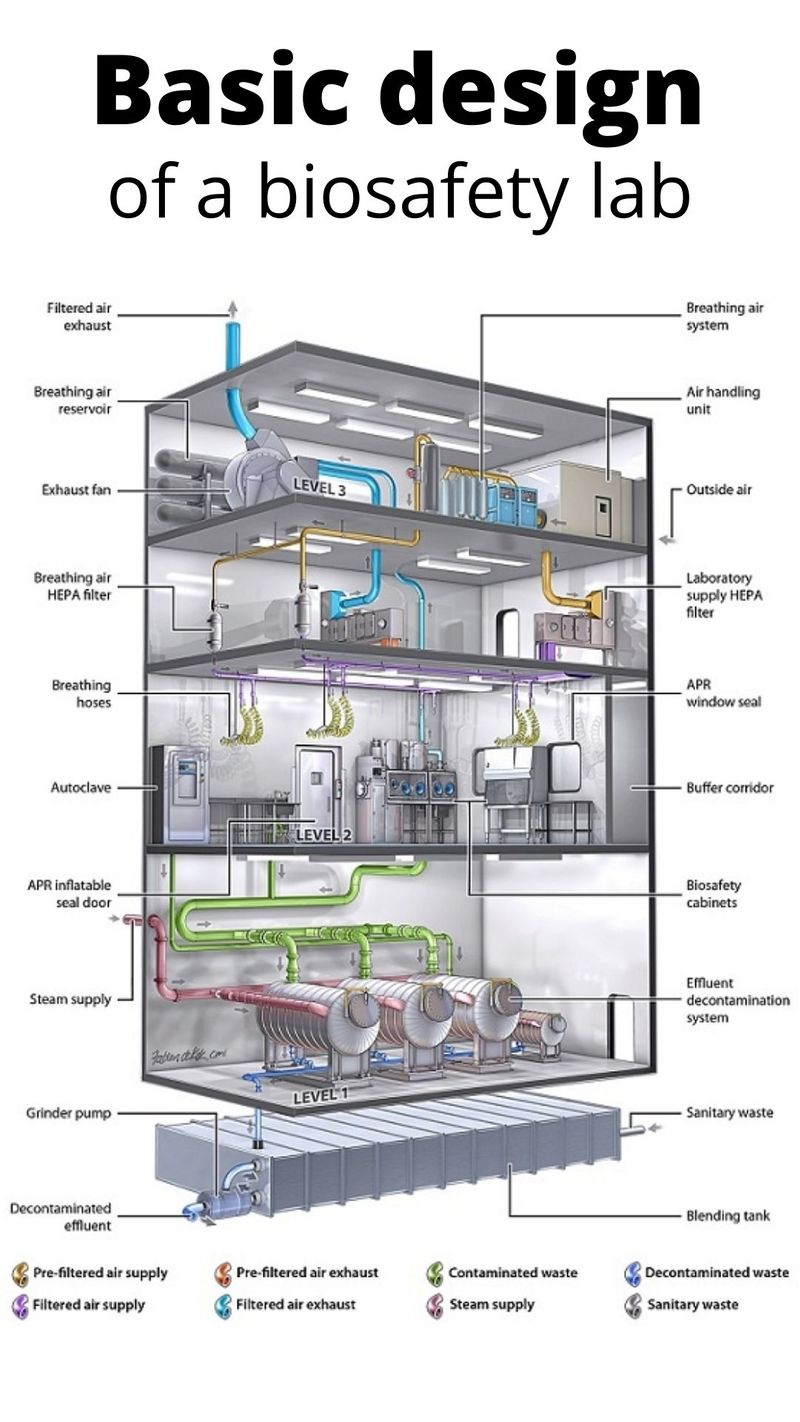
Is there a global system of reporting such accidental lab leaks?
It’s not clear at the moment. A “survey” of biosafety levels 3 and 4 laboratories published in 2016 states that there's no harmonised system for reporting lab incidents and accidents, at least at the European Union level. [https://bit.ly/35kCUfu]. At various levels (national/regional), improved lab safety protocols have minimised lab workers' pathogen exposition while better containment equipment, engineering controls, and safety training contributed greatly to this reduction.
In 2019, US media reported that a deadly germ research was shut down at a US army lab Fort Detrick, Maryland, over safety concerns. Researchers were banned from working with anthrax, Ebola and smallpox at the US Army Medical Research Institute of Infectious Diseases (USAMRIID) until procedures improved. Regulators found problems with disposal of dangerous materials, which led the US government to suspend research at the US military’s leading biodefense centre.
What is the usual route of lab-acquired infections?
About 80% of LAIs are caused by inhalation (particularly by aerosols) or direct contact between contaminated surfaces (gloves and hands). The other routes: percutaneous inoculation (needlestick injuries, broken glass injury, and/or animal bites or scratches) and via smoking, eating, or accidental aspiration through a pipette. But the latter has now disappeared because of banishment of these practices.
What gives lab-acquired infections (LAIs) pandemic potential?
In general, when virologists manipulate microorganisms in the lab, they usually investigate the route of transmission and pathogenicity, specifically the minimal infective dose for humans. Scientists like Harvard's Dr Marc Lipsitch states that one factor that makes a lab “escape” a serious concern is the existence of “super-spreaders”. This makes even a single laboratory-acquired infection into a potential pandemic.
Were there studies conducted labs on pathogens to 'enhance' their function?
In general, the manipulation of microorganisms in labs is the lot of virologists. But in recent years, it has gone a notch higher. In a well-documented field of study, called “gain-of-function” (GOF) research, virologists have focussed on “pathogenicity” or “transmissibility” studies among mammals by respiratory droplets of the following pathogens:
- Influenza
- MERS-CoV
- SARS-CoV-1
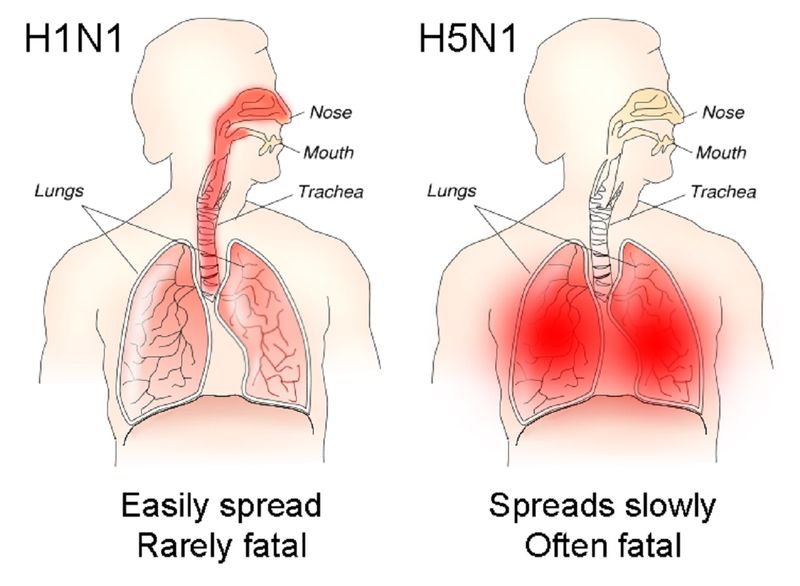
- H5N1
What is GOF research?
GOF studies or research involves experimentation that aims or is expected to (or actually does) increase the transmissibility and/or virulence of pathogens. In general, a GOF research improves the ability of a pathogen to cause disease. GOF research is a subset of “dual-use research” — i.e., research that can be used for both beneficial and malevolent purposes.
Some scientists are gravely concerned with such lab tinkering with infectious diseases.
GOF research: What for?
In general, GOFs are done on pandemic-potential pathogens (PPPs), or viruses or biological agents that may cause a global disease due to a lack of antidote or vaccine. Such research, when conducted by responsible scientists, usually seeks to improve scientific understanding of disease-causing agents and their interaction with human hosts.
In theory, the objectives of GOF studies are three-fold:
- (1) Know of interactions between humans and pathogens;
- (2) Better inform public health and preparedness efforts; and
- (3) Develop medical countermeasures, such as vaccines and/or therapies.
Why are experts are divided over the GOF studies?
Virologists are deeply divided over the benefits of GOF studies. While some push for more GOF research to help humanity, others say there’s “nothing to be gained” from such inherently risky endeavour.
How many BSL-4 labs are there in the world?
BSL-4 is the highest rating (lowest being BSL-1). They have to be built to very high specifications because they deal with the most dangerous pathogens known to science. Usually, those pathogens, if released accidentally, don’t have a known antidote yet. In general, BSL-4 labs have good safety records. There are only a few such labs designated BSL-4. Wikipedia lists over 50 around the world. The authenticity of that list cannot be verified at this point.
Transmissibility: The ability of a disease to be passed on from one person, or organism to another.
How many biosafe laboratories are there in the US?
According to one estimate, the number is about 1,500.
What do we know about coronavirus GOF research funded by US taxpayers?
In 2018, The Lancet reported that in the early part of the previous decade, the “pathogenicity” or “transmissibility” among mammals via respiratory droplets of the influenza virus, the Middle East Respiratory Syndrome coronavirus (MERS-CoV), and Severe Acute Respiratory Syndrome Coronavirus (SARS-CoV-1) had been subject of GOF studies funded by US taxpayers’ money.
In 2014, a ban on US funding for GOF studies was imposed, only to be lifted three years later, in 2017.
On February 1, 2018, more than a year before the SARS-CoV-2 pandemic exploded, scientists remained split over the benefits — and risks — of such studies. By then, a three-year US moratorium on GOF experiments had already been rescinded.
What was the trigger for the funding moratorium?
The situation has its roots in 2011, The Lancet reported. At that time, the US National Science Advisory Board for Biosecurity (NSABB) suppressed two phenotype and genotype studies involving H5N1 viruses that had been modified to allow airborne transmission from ferret to ferret. Ferrets, known scientifically as Mustera potorius furo, are a domesticated form of European polecat.
What triggered the move was the risks seen arising from such studies. A debate raged over “dual-use” life science research, which has largely focused on dangers associated with potential malicious use of such scientific investigations. It’s a bit like nuclear science — which comes with big benefits as well as big risks. Nuclear energy can be used to power huge vessels, entire cities or countries — or trigger a meltdown for the entire planet in a “mutually-assured destruction” shooting war.
Genotype is the genetic constitution of an individual organism.
Was the White House involved in the decision to impose funding moratorium on GOF research?
Yes. On October 16, 2014, the Office of Science and Technology Policy under the Obama White House announced the launch of the US Government (USG) gain-of-function (GOF) deliberative process to re-evaluate the potential risks and benefits associated with certain GOF experiments.
“During this process the USG paused the release of federal funding for GOF studies anticipated to enhance the pathogenicity or transmissibility among mammals by respiratory droplets of influenza, MERS, or SARS viruses,” a White House statement said, then.
The NSABB (National Science Advisory Board for Biosecurity) served as the official federal advisory body on the GOF issue and was tasked with providing recommendations to the USG on a conceptual approach for evaluating proposed GOF research.
When was the recommendation finalised?
In January 2017, the US Government released policy guidance for the review and oversight of research anticipated to create, transfer, or use enhanced pandemic potential pathogens.
What were the concerns raised?
Members of the NSABB board expressed concern that “malign actors” could replicate the work to deliberately cause an outbreak in human beings. The studies were published in full in 2012. US health authorities subsequently issued guidelines for funding decisions on experiments likely to result in highly pathogenic H5N1 viruses transmissible between mammals via aerosols. The guidelines were later expanded to include H7N9 viruses.
The US CDC deems the H7N9 virus has the "greatest potential" compared with other influenza A viruses to cause a pandemic. Currently, the risk is low, because like other type A viruses, it is not easily transmitted between people in its current form.
Any GOF research that makes H7N9 viruses more transmissible and pathogenic would, by definition, raise the risk of turning it into the next pandemic candidate. There are increasing calls for international oversight of such gain-of-function research, and for a more systematic reporting of lab escapes of dangerous pathogens.
One of the most prominent anti-GOF advocates is Harvard University’s Dr Marc Lipsitch, Professor in the Department of Epidemiology at the Harvard T.H. Chan School of Public Health, who is also the Director of the Center for Communicable Disease Dynamics
I still do not believe a compelling argument has been made for why these studies are necessary from a public health point-of-view; all we have heard is that there are certain narrow scientific questions that you can ask only with dangerous experiments. Strains are all different, and we can learn a lot about dangerous strains without making them transmissible.
Lipsitch told a forum in 2015: “I still do not believe a compelling argument has been made for why these studies are necessary from a public health point-of-view; all we have heard is that there are certain narrow scientific questions that you can ask only with dangerous experiments”, he added: "…Strains are all different, and we can learn a lot about dangerous strains without making them transmissible.” He pointed out that every mutation that has been highlighted as important by a GOF experiment has been previously highlighted by completely safe studies.
“There is nothing for the purposes of surveillance that we did not already know”, said Lipsitch. “Enhancing potential pandemic pathogens in this manner is simply not worth the risk.”
What is “dual use” research of concern (DURC)?
The WHO defines DURC as “life sciences research that is intended for benefit, but which might easily be misapplied to do harm”. A study published in Nature Reviews Microbiology in 2014 pointed to recent studies, particularly those on influenza viruses, that have led to renewed attention on dual-use research of concern (DURC).
On December 19, 2017, the US National Institutes of Health (NIH) announced that they would resume funding gain-of-function (GOF) experiments involving influenza, MERS and SARS-CoV-1). That move effectively ended a 3-year moratorium.
When was the funding ban lifted?
On December 19, 2017, the US National Institutes of Health (NIH) announced that they would resume funding gain-of-function (GOF) experiments involving influenza, MERS and SARS-CoV-1). That move effectively ended a 3-year moratorium. [Source: https://bit.ly/3pUNIdL]
How many biosafe laboratories are there in the US? According to one estimate, the number is about 1,500.
Is there a way to track safety breaches or laboratory “escapes”?
It’s a good question; the answer is problematic. Statistics on the number of breaches in those 1,500 or so high-containment laboratories in the US are hard to come by, say experts. Though “serious” events (ones that result in an infection in the community are virtually unknown) are rare, any escape is a big blot on a lab technician’s or researcher’s career. It can bring an entire house (laboratory or the insitution behind it) down in terms of reputation. Therefore, there’s a built-in disincentive for reporting incidents rooted in the human psyche and self-preservation instinct.
Given the history of leaks and lab escapes of dangerous pathogens, it’s no longer just a question of if — but when. And when an outbreak spreads to the community, it can bring untold consequences.
There's one problem with lab-acquired infections: though stricter lab safety protocols are mandated, no rule or legislation can prevent it. A single escape or human error could lead to a situation horribly worse than any breakout movie. In virology, when it comes to pandemic-potential pathogens, there’s a general rule: infection begets transmission, transmission begets epidemic, and epidemic begets pandemic.








