
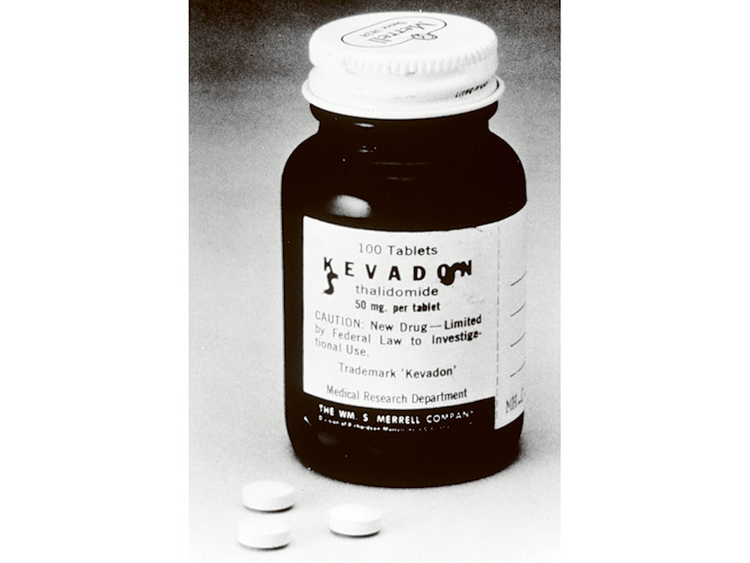
New York: The sedative was Kevadon, and the application to market it in the United States reached the new medical officer at the Food and Drug Administration in September 1960. The drug had already been sold to pregnant women in Europe for morning sickness, and the application seemed routine, ready for the rubber stamp.
But some data on the drug’s safety troubled Dr Frances Oldham Kelsey, a former family doctor and teacher in South Dakota who had just taken the FDA job in Washington, reviewing requests to licence new drugs. She asked the manufacturer, the William S. Merrell Co. of Cincinnati, for more information.
Thus began a fateful test of wills. Merrell responded. Kelsey wanted more. Merrell complained to Kelsey’s bosses, calling her a petty bureaucrat. She persisted. On it went. But by late 1961, the terrible evidence was pouring in. The drug — better known by its generic name, thalidomide — was causing thousands of babies in Europe, Britain, Canada and the Middle East to be born with flipperlike arms and legs and other defects.
Kelsey, who died on Friday at the age of 101, became a 20th-century American heroine for her role in the thalidomide case, celebrated not only for her vigilance, which spared the United States from widespread birth deformities, but also for giving rise to modern laws regulating pharmaceuticals.
She was hailed by citizens’ groups and awarded honorary degrees. Congress bestowed on her a medal for service to humanity and passed legislation requiring drugmakers to prove that new products were safe and effective before marketing them. President John F. Kennedy signed the landmark law that she had inspired, and presented her with the nation’s highest federal civilian service award.
“Her exceptional judgement in evaluating a new drug for safety for human use has prevented a major tragedy of birth deformities in the United States,” Kennedy said at a White House ceremony.
Kelsey was appointed to the Order of Canada last month and presented with the honour in a private ceremony the day before her death at her daughter Christine Kelsey’s home in London, Ontario, where Frances Kelsey had been living, according to John Swann, a historian at the FDA and a friend of hers.
In 1962, the FDA set up a branch to test and regulate new drugs, and Kelsey was put in charge of it. Later, she became director of the agency’s Office of Scientific Investigations, and in a distinguished 45-year career with the FDA helped rewrite the nation’s medical-testing regulations, strengthening protections for people and against medical conflicts of interest. The rules have been adopted worldwide.
She was born Frances Oldham in Cobble Hill, on Vancouver Island in British Columbia, on July 24, 1914, one of three children of Frank and Katherine Stuart Oldham.
Frances attended schools in Victoria and McGill University in Montreal, earning a bachelor’s degree in 1934 and a master’s in science in 1935. In 1936, she enrolled at the University of Chicago, where she earned a doctorate in pharmacology in 1938. She joined the faculty that year and became an assistant professor of pharmacology.
She and Dr Fremont Ellis Kelsey, a professor in the university’s pharmacology department, married in 1943. He became a special assistant to the surgeon general in 1962 and died in 1966. The couple had two daughters.
Besides her daughter Christine, Kelsey is survived by another daughter Susan Duffield, two grandsons and a sister.
Kelsey received her medical degree from the University of Chicago in 1950. She was an editorial associate for the American Medical Association Journal in Chicago for two years before the Kelseys moved to Vermillion, South Dakota. He became chairman of physiology and pharmacology at the University of South Dakota medical school. From 1954 to 1957, she taught pharmacology there, and for the next three years was in private medical practice. She became a naturalised US citizen in 1956.
Her husband’s appointment to a post at the National Institutes of Health took the family to Washington in 1960. She accepted the FDA job evaluating applications for licenses to market new drugs. Merrell’s was one of the first to cross her desk.
Glowing claims
The company made glowing claims for Kevadon’s safety and effectiveness. It had been developed in West Germany, and since 1957 had been widely sold in Europe and elsewhere as an excellent sedative, Merrell said, giving prompt, deep, natural sleep without hangovers. Moreover, doctors had recently been prescribing it to women to suppress nausea in pregnancy.
Laws governing new drugs had been on the books for decades but were not always rigorously enforced, and FDA approval was often routine. But Kelsey, working with a chemist and a pharmacologist, found the evidence for Merrell’s claims about Kevadon to be insufficient. She withheld approval and asked Merrell for more data on toxicity, strength and purity.
Merrell stood to make millions and was anxious to get moving. It had tons of Kevadon in warehouses, ready for marketing, and 1,000 US doctors had already been given samples for “investigational” research. The company supplied more data, but also mounted a campaign to pressure Kelsey. Letters, calls and visits from Merrell executives ensued. She was called a fussy, stubborn, unreasonable bureaucrat.
But she refused to be hurried, insisting that there was insufficient proof. In February 1961, she read a letter in The British Medical Journal from a doctor who suggested that thalidomide might be causing a numbing condition in arms and legs. She notified Merrell, and the company began its own inquiry. In May, she told Merrell that the drug might affect the limbs of foetuses. The company called the evidence inconclusive.
“I had the feeling,” she wrote after a meeting with company executives, “that they were at no time being wholly frank with me, and that this attitude has obtained in all our conferences, etc, regarding this drug.”
Six months later, European reports indicated the drug was linked to an epidemic of phocomelia, a rare but monstrous malformation of limbs in newborns. Merrell withdrew its application as reports of the births of “thalidomide babies” came in from many countries. Kevadon samples given to US doctors were traced, but not all were retrieved. About 40 births of deformed babies in the United States were reported.
Enzymes
Eventually researchers learnt that thalidomide crossed the placental barrier and retarded development of the foetus, whose drug-metabolising enzymes are undeveloped. No one knows how many babies were affected by thalidomide, but estimates range into the tens of thousands in Europe alone. Many were born without arms or legs, some with no limbs or with withered appendages protruding directly from the trunk. Some had no external ears or deformities of the eyes, the oesophagus or intestinal tracts.
After an article in The Washington Post led to global coverage, Kelsey was hailed as a hero. She insisted that her pharmacologist, Oyam Jiro, and chemist, Lee Geismar, as well as her superiors share the credit. But attention focused on her partly because the Kennedy Administration and its allies in Congress wanted to use the case to pass stronger drug regulations. The 1962 law required tighter proof of the safety and effectiveness of new drugs, full disclosure of side effects and generic names, and swift removal of unsafe drugs from the market.
When she became widely known, Kelsey, a tall, greying woman who spoke softly and never wore cosmetics, seemed modest to the point of shyness. But she testified in Congress, spoke to women’s groups and at college forums and gradually became accustomed to the spotlight.
In 2000 Kelsey was inducted into the National Women’s Hall of Fame, joining the ranks of Helen Keller, Eleanor Roosevelt, Margaret Mead and other luminaries. She retired in 2005, and in 2010 was honoured by Dr Margaret Hamburg, then the FDA commissioner, as the first recipient of an award that continues to be given annually in her name.
— New York Times News Service










_resources1_16a3106a819_small.jpg)

