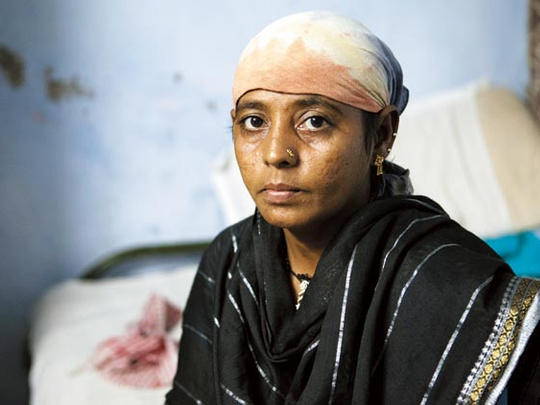
At first Hinaben Girish dismissed her headache, after all, she had gone through this the week before and it had subsided. But the pain grew more intense. “My skull felt like it was splitting,” the mother of three says. “I could have tried taking some painkillers, but I didn’t want to take any more tablets.” That’s because Hinaben, 38, had been taking four tablets a day the previous week as part of a drug trial – the latest in a long line she’d done to raise money to realise her dream.
“I dreamt of owning my own home and giving my children a good education and this was my way of financing it,” she says.
The Rs5,000 (Dh400) she was paid at the end of every three-month trial by a pharmaceutical company near her home in Ahmedabad, western India, meant she could save up enough to buy a small house.
She did not know what the latest tablets she was testing were for, “but I would get a headache as soon as I took them,” Hinaben says.
When she passed out, her husband Girish Bhai, 40, rushed her to a nearby government hospital where tests showed she was suffering from astrocytoma grade 2 – a type of cancer of the brain.
Her doctor has suggested it could have been caused by an unknown drug that was tested on her during a clinical drug trial in 2011.
She is undergoing chemotherapy at present but doctors cannot be sure she will survive or make a full recovery.
Now, lying in her cramped one-room rented accommodation, Hinaben is left with her dreams and health in tatters and is scared for the future of her family. She says, “My life has been ruined. If only I’d known more about this industry before I got involved... Now, I can’t fight these companies. I can do nothing.’’
Hinaben is just one among hundreds of volunteers in India who line up outside pharmaceutical companies and contract research organisations (CROs) or outsourcing units to earn easy money taking part in clinical drug trials. They normally hear of these trials through word of mouth and agents who come to their villages offering the poor – often illiterate – men and women a chance to earn “a lot of money quickly’’.
For more than three years, Hinaben was a regular at one such company’s clinic. While some trials lasted around 40 days, others carried on for about three months and most of the time Hinaben, eager to make as much as she could quickly, took trials back to back. The companies conducting the trials never objected to this. She was so pleased with her experience and regular income she even went on to encourage several other women from her area to become volunteers like her. “I used to tell them about the benefits of becoming a volunteer. The money I was getting was good and it was changing our lives. I was able to send my children to a good school and could even hope to own a house soon. Also, I didn’t have any side effects until the headaches about two years ago,’’ she says.
While undergoing the earlier trials, Hinaben had been told the drugs were being tested for use in treating gastric and cardiac ailments and certain skin diseases but little more.
Easy access, low costs and relaxed rules
Many pharmaceutical companies are based in Ahmedabad, the largest city in Gujarat. They are involved in outsourcing work from a number of multinational pharmaceutical companies that attract hundreds of people, many of them with large families to take care of.
Some of the participants in the drug trials have been quoted as saying they agreed to take part simply because of the recommendation of their doctor who was very often the very person conducting the trials.
Clinical trials are important in a drug’s development cycle and constitute tests that help companies understand the safety and efficacy of a drug as well as monitor adverse effects. India, which has earned the sobriquet of “the developing world’s pharmacy”, has fast become one of the most popular destinations for multinational drug companies to test products thanks to the large population, easy availability of people with conditions such as diabetes, cancers and asthma, skilled clinical research professionals, favourable laws and a large pool of central labs. All these factors act as magnets to the $18-billion (Dh66-billion) or so global contract research organisation industry.
According to a report in the Washington Post at the beginning of last year, the number of Indians participating in drug trials shot up from near zero to 150,000 since India eased guidelines for conducting drug trials in 2005, with international drug companies taking advantage of lower costs there. Analysts say conducting drug trials in India saves multinational companies almost 40 per cent of the total cost of drug development because health-care professionals are cheaper and liability is not very high.
The Indian domestic drugs trial market was worth about $485 million in 2011, according to a Reuters report. Predictions are that it could double to $1 billion by 2016.
Inability to read the agreement
Attracted by the opportunity to earn quick money, thousands of poor people enter into contracts made by drug companies agreeing to become guinea pigs little knowing that their health and often life itself could be at risk.
Hinaben is illiterate and had never held a job before. “I’d always wanted to buy a house,’’ says the housewife, adding drug trials appeared to be too tempting to ignore. “Girish only earns Rs3,500 a month, which is just enough to support us and his mother. It would have taken us years of saving to buy a small house.’’
So in 2008, Hinaben decided to offer her services to drug trial clinics. While she did not know what medicines she was taking, she says she had been told of possible health risks but not in any great detail, and Hinaben says she felt too intimidated to probe for details.
Her husband knew many volunteers in his circle of friends and supported Hinaben’s decision. “We needed the money and as he had seen how the fortunes of his friends had changed he was enthusiastic about going for it. We knew no one who had been ill because of it,’’ says Hinaben.
She admits that she was initially scared but in the end the benefits outweighed the negatives. “It’s minimal work so I could still be at home with my children – son Paras, 17, and daughters Bhumika, 15 and Reshma, 14 – and it looked like a decent way to make money. Also, the people at the centre insisted there was no danger so I agreed,’’ she claims.
Hinaben remembers that she was told to sign a document, which in her enthusiasm to get started she blindly did, unable to read it herself and not bothering to get it read by anybody. The papers were retained by the company and she was not given a copy to take home. “I don’t know what was written in the papers,’’ she says.
Initially, she was asked to provide a blood sample and once that was tested and it was found she did not have any major health problems, she was taken on.
The research organisation then gave her a set of tablets and she was told to consume a fixed number every day over a period of a week and then report to the centre for blood tests. She says she was asked to provide blood samples five to ten times in a three-month period for one particular drug trial.
“I participated in 17 trials, all for the same company, in less than four years and was paid Rs5,000 for each trial. Although the payments were late and sometimes were less than what was promised, it was all fine. It was going well and I had no side affects or ill health.’’
With the money she earned from the trials she sent her children to an English-medium school and it seemed the dream of owning a house would soon become a reality. But the optimism ended when Hinaben began to suffer severe headaches in late 2011 after she was given a set of tablets to have as part of the trial.
“As soon as I started taking the tablets my head started aching. It felt like it was going to burst. When I informed the drug company about this, I was told not to take any medicine for pain relief as it would interfere with their tests.”
As she herself didn’t want to take any more medication, Hinaben says, “I battled with the pain for almost 48 hours while the clinic continued to take blood samples for their trials. They didn’t care too much about my head pains.’’
A week after she experienced the first bout of headaches, Hinaben went to the drug trials clinic where she was given a second set of the same tablets and as soon as she started taking them she began experiencing severe headaches again.
“It was worse than before. I left the clinic and when I reached home I vomited and fell unconscious. I opened my eyes again in hospital five days later,’’ she says.
Since then, Hinaben has spent almost all her savings on hospital fees. While initially the doctors who were treating her for her headaches did not know that she had been subjected to drug trials, Hinaben eventually told them.
She approached the contract research organisation asking it to pay her hospital bills, but it bluntly declined, she says. “In fact they did not even pay for the last trial and insisted there was no proof [of a connection] between the trial and my ill-health,’’ she says, adding that she does not have the money to take on the big companies in court.
Opposition mounts
Indian health minister Gulam Nabi Azad told his parliament last year that between 2010 and mid-2012, a total of 1,317 people had died due to clinical trials in India.
The Supreme Court of India has criticised the government saying “uncontrolled clinical trials are causing havoc to human life”.
Activists allege that the companies treat volunteers as if they are disposable. Often the volunteers are not told about the health risks or allowed to know details of the medicines they consume. Most of the volunteers are illiterate and do not even understand the consent forms and other documents they are made to sign wherein they effectively waive all their rights.
Swasthya Adhikar Manch (SAM), an activist group fighting against the unethical practices by pharmaceutical companies, has filed a petition in an Indian court demanding more government regulations.
Amulya Nidhi, a SAM spokesperson says, “These trials are conducted in India either because they would not be allowed anywhere else or because such trials are extremely expensive to conduct in other countries. The poor, illiterate and vulnerable sections of the society in India become subjects of these illegal clinical trials.’’
Activists say the companies not only compromise the lives of the volunteers but also the results of the drug trials by engaging them in multiple trials at one time to complete the projects quickly and to cut costs. They also allege more women are getting involved in the drug-trial industry compared to men, since it requires little time away from home.
The government is planning to amend existing laws and regulate the drug-testing industry. It is considering amending the Drugs and Cosmetics Act to make lapses by pharmaceutical multinational corporations a punishable offence under law. The revised Act would also put specific responsibility on investigators and sponsors of drug trials, making it mandatory for them to provide medical help to the subjects involved in trials, and in case of serious adverse events like death.
The drug controller general of India, GN Singh early this year said that following a Supreme Court ruling revoking the powers of the Central Drugs Standard Control Organisation, the nodal agency for monitoring clinical trials in India, no new clinical trials would be approved in India until the health ministry defines a regulatory regime for such tests. The government also aims to set up independent ethics committees under medical institutes to monitor ongoing drug trials in India, reports from India say. The move is to combat irregularities in such trials.
The recent spate of criticism over the functioning of drug-trial clinics has raised serious fears about the future of drug development in the country.
When asked to comment on the current spate of criticism about the drug trials, Rahul Nijhawan, associate director of global project management at Cliantha Research Limited, a clinic that conducts trials in Ahmedabad, says, “We have been approved by local and international bodies and we always follow local laws and ethics when conducting drug trials.’’
An agent’s regrets
Ravi Keshwani, 42, from Vinobabhave Nagar, in Ahmedabad, was a former drug-trial subject before he became an agent for CRO in Ahmedabad. He says he introduced more than 800 volunteers to drug trial clinics in the past five years. But now he severely regrets it. He earned around Rs1,000 per volunteer, but he always felt bad about the way the companies treated them. He participated in a trial twice himself before becoming an agent.
He says being an agent is “a double-edged sword. If anything goes wrong or if there are any problems, agents are held responsible by both the companies and the volunteers.’’
Ravi says poverty is driving people to risk their lives at the clinics, which according to him adopt very unethical practices to save the cost on studies. He says, “If companies follow rules, they couldn’t complete a single project in three months, but working as they do, they complete the same projects in 20 to 25 days.’’
According to him, clinics conduct trials on medicines used for treating cancer, depression, blood coagulation, urinary diseases, nervous system diseases, cardiac, reproductive disorders and other ailments. “The volunteers often complain of kidney problems, bleeding, vomiting, depression, nervous disorders, headaches, body aches, joint pains and even cancer after participating in the trials,’’ he adds.
He says like most volunteers, he joined the industry to make a living but has become disillusioned by the attitude of the clinics. “I now feel guilty about exposing volunteers to dangers so I have decided to fight against these clinics. They need to adapt their practices as these are people’s lives they are dealing with.’’
Hinaben too regrets ever getting involved in pharmaceutical drug trials but insists the money was always too tempting to turn down. “If there’s a way to earn money for a better future then you do [what they want you to]. These companies know we are desperate to earn money.’’
However if the proposed amendments to laws come into force, there will be a lot more transparency and volunteers’s rights will be protected when they sign the contract, say analysts.
*Additional input by Anand Raj OK.